physical
advertisement

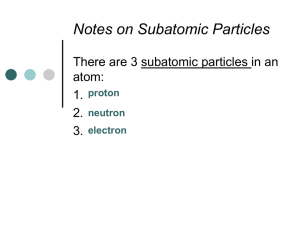
Unit 2 – Section A Why we Use What We Do HW 1 Read & take notes on sections A.1 (pg . 110) & A.2 (pg. 111) , being certain to address all the examples in section A.2 A.1 – Properties Make the Difference Physical properties: » Color » Density » Odor May be determined without altering chemical make-up Ex. 8.96 g/cm3 2.06 g/cm3 A.1 – Properties Make the Difference (continued) Physical change: » Melting » Boiling » Bending An actual physical change, material unaltered Ex. A.1 – Properties Make the Difference (continued) Chemical change is when a material changes into one or more new substances. A.1 – Properties Make the Difference (continued) Chemical properties determine a chemical’s usefulness. A.2 – Physical & Chemical Properties S.P. 1: Copper compounds are often blue in color PHYSICAL PROPERTY S.P. 2: Oxygen gas supports the burning of wood CHEMICAL PROPERTY A.2 – Physical & Chemical Properties (continued) 1: Pure metals have a high luster (shiny) PHYSICAL PROPERTY 2: The surface of some metals become dull when exposed to air CHEMICAL PROPERTY A.2 – Physical & Chemical Properties (continued) 3. N2 which is a relatively nonreactive element at room temp. , can form NO2 at high temperatures of an auto engine. CHEMICAL PROPERTY 4. Milk turns sour if left too long at room temperature CHEMICAL PROPERTY A.2 – Physical & Chemical Properties (continued) 5. Diamonds are hard enough to be used as coating for drill bits. PHYSICAL PROPERTY 6. Metals are typically ductile (can be drawn into wires) PHYSICAL PROPERTY A.2 – Physical & Chemical Properties (continued) 7. Leavened bread dough increases in volume if it is allowed to rise before baking. CHEMICAL PROPERTY 8. Unreactive argon gas, rather than air, is used to fill many light bulbs to prevent the metal filament wire inside the bulb from being destroyed through oxidation. CHEMICAL PROPERTY A.2 – Physical & Chemical Properties (continued) 9. Generally, metals are better conductors of heat and electricity than are nonmetals. CHEMICAL PROPERTY HW 2 Read & take notes on sections A.3 & A.4. And complete the following Element Cards A.3 – Properties Matter: Designing the new coin… Every element has its own physical & chemical properties. When deciding what to use how do we decide the best substance for our needs? 1. 2. 3. 4. 5. 6. 7. 8. 9. A.4 – The Chemical Elements How are chemical elements placed on the periodic chart of elements? A.4 – The Chemical Elements (continued) Two major classes of elements are metals and nonmetals. Several elements have properties of both and are called metalloids. HW 3 Pre-read section A.5 A.5 – Metal or Nonmetal? See pgs 115 – 117 By the end of class – answer how you did on the predictions AND questions 1-4 on pg 117. A.5 – Metal or Nonmetal? (Lab follow up) Common lab issues: 1) Make certain you answer the question being asked. 2) Restate the question in the form of a statement and ADD your information. 3) Make it a goal to show me that you have actually thought about the question, rather than prove you never even considered it. (example next slide) No shoveling please . . . Q – How did you do on your predictions? (tell me below) Student answer: “On my predictions I made the common sense answer but there was a few more per question and a few surprising ones but relatively well.” As apposed to the following Q – How did you do on your predictions? (tell me below) Student answer: “Malleable * I got 2/4 correct. Me – Mg & Zn, results Al, Cu , Mg ,Zn HW 4 Pre-read and take notes on A.6 And make certain you have your element cards with you when you come to class next day. A.6 – The Periodic Table (A Brief History) By mid-1800s, chemists identified ~60 elements. Five were nonmetals, gasses at room temperature. hydrogen (H) oxygen (O) nitrogen (N) fluorine (F) chlorine (Cl) Two liquids were also known. Metal : mercury (Hg) Nonmetal : bromine (Br) Scientist tried to place elements near one another based on similar properties. A.6 – The Periodic Table (A Brief History) Dimitri Mendeleev, a Russian chemist published one of the first such tables in 1869. A.6 – The Periodic Table (continued) An offshoot is still used today based on two characteristics: 1) atomic masses and 2) combining capacity. A.7 – Grouping the Elements See page 119 HW 5 Read & take notes on sections A.8 A.8 – The Pattern of Atomic Elements Refresher : Atoms consist of sub-atomic particles Protons , small positively charged particles and neutrons, particles with no charge make up the nucleus of an atom. Electrons are a negatively charges particles found in varying energy levels surrounding the nucleus. A.8 – The Pattern of Atomic Elements (continued) The number of protons in an atom is called the atomic number. For the example to the right the value would be _____ Mass number is the total number of protons and neutrons in the nucleus of an atom. Example to the right the value would be ____ A.8 – The Pattern of Atomic Elements (continued) While all atoms of particular elements have the same number of protons, they contain different number of neutrons. Example (from book) , carbon (C) always has 6 protons, but may contain 6,7, or 8 neutrons – these are called isotopes. A.8 – The Pattern of Atomic Elements (continued) So why are the atomic masses we see on the periodic table not integers (whole numbers)? They take into account the weighted average of the atomic masses of the naturally occurring isotopes. Candium Isotopes HW 6 Pre-read section A.9 A.9 – Periodic Variation in Properties See pgs 122-123 Graphing lab we’ll do together. After graphing the data , please answer questions 1,3 & 4 on pg 123 HW 7 Read & take notes on sections A.10 and answer A.11 A.10 – Organization of the Periodic Table The table forms horizontal rows called periods. Their periodic relationship is seen in the modern table shown below. A.10 – Organization of the Periodic Table (continued) Each of the columns is called a group or family, due to their similar properties A.10 – Organization of the Periodic Table (continued) The alkali metal family is the six (6) elements in the first column starting with Lithium A.10 – Organization of the Periodic Table (continued) The noble gas family is the six (6) elements in the rightmost column which are unreactive, chemically inert elements. A.10 – Organization of the Periodic Table (continued) The halogen family is the five (5) elements immediately to the left of the nobles that readily form ions. A.11 – Predicting Properties Using the periodic chart we can predict properties of the various elements. A.11 – Predicting Properties (continued) 1) Given, the b.p. of argon (Ar) is -186°C and of xenon (Xe) is -112°C. Estimate the b.p. of krypton (Kr). Krypton should have a boiling point between argon 87 K and xenon 161 K of about 124 K – actual = 120 K. A.11 – Predicting Properties (continued) 2a) Given, the m.p. of potassium (K) is 337°K and of cesium (Cs) is 302°K. Estimate the m.p. of rubidium (Rb). Actual = 312 K 2b) Would the m.p. of sodium (Na) be higher or lower than (Rb) , explain. A.11 – Predicting Properties (continued) 3) Known, silicon tetrachloride exists (SiCl4) Predict the formula for Ge and Cl. GeCl4 A.11 – Predicting Properties (continued) 4) Known, the following exist : NaI, MgCl2, CaO, Al2O3, and CCl4 Predict the combination a) C and F CF4 A.11 – Predicting Properties (continued) 4) Known, the following exist : NaI, MgCl2, CaO, Al2O3, and CCl4 Predict the combination b) Al and S Al2S3 A.11 – Predicting Properties (continued) 4) Known, the following exist : NaI, MgCl2, CaO, Al2O3, and CCl4 Predict the combination c) K and Cl KCl A.11 – Predicting Properties (continued) 4) Known, the following exist : NaI, MgCl2, CaO, Al2O3, and CCl4 Predict the combination d) Ca and Br CaBr2 A.11 – Predicting Properties (continued) 4) Known, the following exist : NaI, MgCl2, CaO, Al2O3, and CCl4 Predict the combination e) Sr and O SrO HW 8 Read & take notes on sections A.12 A.12 – What Determines Properties? Major difference between metals and nonmetals is that metal atoms lose electrons more easily. A.12 – What Determines Properties? (continued) Some physical properties of metals depend on attractions among their atoms. Stronger attractions = higher melting points m.p. of Mg is 650°C m.p. of Na is 98°C A.12 – What Determines Properties? (continued) Understanding properties of the chart is the key to predicting – this allows chemists to create new chemical compounds to meet specific needs. Predicting Properties Periodic Chart of Elements Online Link http://www.ptable.com/ HW 9 See the next slide A.13 – It’s Only Money Address questions 1-6 on pg 127.