슬라이드 1
advertisement
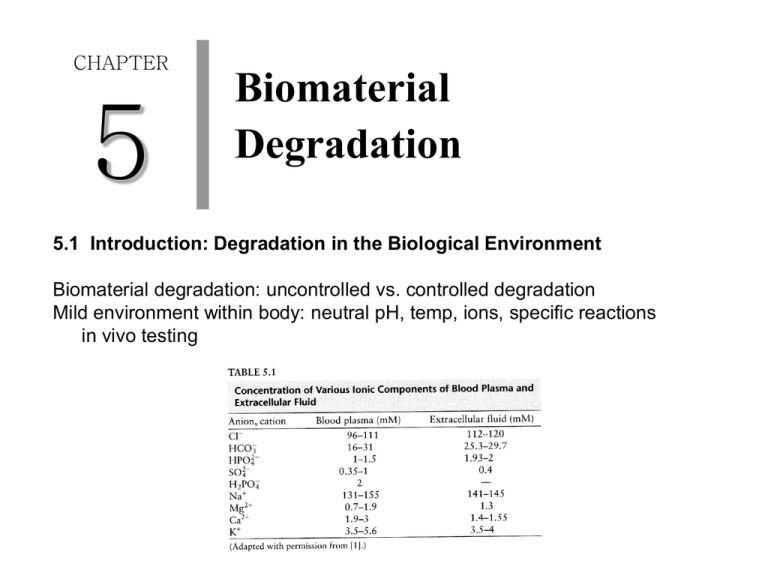
CHAPTER 5 Biomaterial Degradation 5.1 Introduction: Degradation in the Biological Environment Biomaterial degradation: uncontrolled vs. controlled degradation Mild environment within body: neutral pH, temp, ions, specific reactions in vivo testing 5.2 Corrosion/Degradation of Metals and Ceramics Metal degradation: corrosion --- metal durability within body / biocompatibility 5.2.1. Fundamentals of Corrosion (1) Oxidation-reduction reactions corrosion: electrochemical process & electron transfer Anode: oxidation reaction Cathode: reduction reactions 1) in acidic milieu 2) oxygen in acidic solution 3) oxygen in neutral or basic solution Battery: electrochemical (galvanic) cell two metal strips salt bridge wire connection with voltmeter (2) Half-cell potentials standard reduction potential (electron affinity) with standard hydrogen electrode (H2 gas through 1M HCl with platinum) actual reduction potential: Nernst equation galvanic series metals towards the bottom of Table 5-3 : easy oxidation (unstable, degradation) (3) Nernst equation temp & conc. of metal ions (4) Galvanic corrosion two different types of metals in the body connected via physiological fluid more active metal (oxidation, anodic) complex corrosion process oxidation rate = reduction rate slow overall corrosion (passivation) 5.2.2. Pourbaix Diagrams and Passivation Pourbaix diagram: regions of corrosion and non-corrosion as a function of cell potential and pH 1) corrosion 2) immune: cathode protection 3) passivation: surface oxidation --- stable solid film (oxide or hydroxide) no rate prediction rate = current flow / surface area other factors involved 5.2.3. Contribution of Processing Parameters enhanced corrosion in vivo microstructure w/in implant ---- change in localized ion conc. ---- corrosion rate (1) Crevice corrosion at a narrow, deep crack O2 depletion anodic reaction in the crevice O2 reduction (pH increase) Cl- influx insoluble hydroxide & H+ liberation pH decrease (2) Pitting corrosion flaw or disrupted passivation film small anode and large cathode 1) inequality in surface area 2) rates of the redox reactions dissolution of anodic regions (3) Intergranular corrosion grain boundaries --- high energy --- more active region [intergranular attack] 5.2.4. Contribution of the Mechanical Environment location of implants --- corrosion rate (1) Stress & Galvanic corrosion bending ---- tensile side (anodic) & compressed side [galvanic corrosion] (2) Stress corrosion cracking metals under both tension and corrosive environment small cracks ---- crack propagation and brittle fracture (3) Fatigue corrosion continuous loading --- disruption of passivating film --- surface exposure [corrosion] fatigue cycle, corrosion fatigue, premature device failure (4) Fretting corrosion motion near the implant (not by loading) removal of the metal’s passivating layer [nick on the surface] 5.2.5. Contribution of the Biological Environment biological milieu ---- corrosion rate in vivo (1) inflammatory cells pH and strong oxidizing agents growth of the passive layer (2) proteins 1) protein binding to metal surface 2) proteins as electron carriers 3) metal binding proteins [equilibrium favoring metal dissolution] (3) bacteria device infection by-products H2 consumption and anodic dissolution 5.2.6. Means of Corrosion Control (1) devices with few stress raisers (2) combination of metals (located close in galvanic series) (3) non-reactive metals and metals with passive oxide coating (4) heat-treated stainless steel (5) nitric acid pre-treatment (6) surface coating 5.2.7. Ceramic Degradation passivating layer 형성 more stable in physiological environment (ionic character) inert / resorbable / controlled surface reactivity ceramic degradation under mechanical environment stress induced degradation ceramic porosity [stress raiser & surface area 증가] 5.3. Degradation of Polymers polymer degradation in the body rate of polymer degradation 5.3.1. Primary Means of Polymer Degradation (1) swelling/dissolution [plasticizer] 1) ductility 2) crystallinity 3) mechanical & thermal properties (Tg) 4) complete dissolution in aqueous environment (2) chain scission bond rupture ---- molecular weight 감소 hydrolysis / oxidation 5.3.2. Chain Scission by Hydrolysis Hydrolysis 1) reactivity of groups of the polymer backbone 2) extent of interchain bonding 3) amount of media (water) Susceptibility to hydrolysis 1) a large number of cleavable groups 2) hydrophilic domains for water influx 3) low initial mol. wt. and low X-link density 4) low or no crystallinity 5) Tg < body temp 6) high surface area to volume ratio 5.3.3. Chain Scission by Oxidation (1) Oxidation via highly reactive species homolysis vs. heterolysis extent of oxidative degradation 1) number of susceptible chemical domains 2) lower mol wt polymers 3) less tight X-linking (2) Metal-catalyzed oxidation metal corrosion ---- strong oxidizing agents ---- attack polymer coating 5.3.4. Other Means of Degradation (1) Environmental stress cracking (stress corrosion cracking in metals) polymer ---- tensile stress ---- deep crack on the exterior ---- fracture (2) Enzyme-catalyzed degradation enzymes --- affinity for certain chemical groups in polymer ---- catalysts hydrolytic or oxidative degradation 5.3.5. Effects of Porosity pores --- stress raiser (mechanically induced degradation) --- surface area (more space for cleavage) 5.4. Biodegradable Materials controlled biomaterial degradation temporally nature of the material (tissue engineering, drug delivery) biodegradation / bioerosion 5.4.1. Biodegradable ceramics calcium phosphate --- calcium hydroxyapatite & tricalcium phosphate (hydrated calcium sulfate or bioactive glasses) (1) Erosion mechanisms solubility, local pH, dissolution of grain boundaries (2) Factors that influence degradation rate 1) chemical susceptibility of the material 2) crystallinity 3) amount of media (water) 4) surface area / volume ratio 5) high mechanical stress 6) pH drop 5.4.2. Biodegradable Polymers (1) Introduction to biodegradable polymers and definitions Hydrolytic degradation of polymers 1) bulk degradation water penetration rate > polymer degradation rate [cracks and fissures] 2) surface degradation water penetration rate < polymer degradation rate [implant thickness 감소, with mechanical integrity maintained] no good integration with the surrounding tissue in vivo (2) Degradation mechanism 1) breaking X-linked bonds between water-soluble polymer chains 2) cleavage of hydrophobic side chains to reveal hydrophilic groups 3) cleavage of polymer backbone (3) Factors that influence degradation rate enzyme degradation 1) amount of enzyme 2) number of cleavable moieties hydrolytic degradation 1) reactivity of chem groups 2) bonding between chains 3) amount of media 4) surface area 5.5 Techniques: Assays for Extent of Degradation in vitro and in vivo testing signs of degradation 1) mass loss 2) different physical and chemical properties 3) visual inspection --- color and crack formation 4) material surface with light and electron microscopy corrosion test for metallic implants in vitro 1) electrochemical testing IV-curve 분석







