Smelly Balloons
advertisement

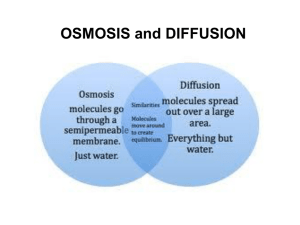
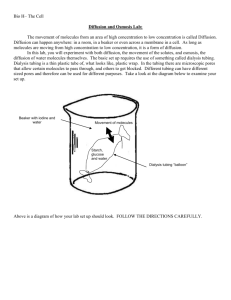
Diffusion RS: Ms. Alvarez CT:Mrs. Rojas Adapted from C. Goedhart Stinkbombs Why does the smell of a stinkbomb travel? Why does the smell eventually disappear? Why do smells linger longer in a small room than in a large room? Diffusion • Molecules move from an area where there are many to an area where there are few. – This is called moving down a concentration gradient. • Molecules move until they are evenly distributed. – Even distribution is called equilibrium. • Molecules can diffuse in a liquid or in a gas. Solutions • A solution is a mixture of two or more substances. • Solute: a substance (like salt) that breaks up in a solvent (like water). • Solvent: a liquid with the power to break apart (dissolve) substances. Solute size • If there are no barriers, a solute of any size eventually reaches equilibrium (or even distribution) in the solvent. • Cell membranes let small solutes in and out through diffusion. • Larger solutes (or molecules) can’t diffuse into the cell, and enter through special proteins or vesicles. Osmosis • Osmosis is a kind of diffusion that refers to the movement of water. • Water likes to move from an area where there are few solutes to an area of many solutes. • In other words, water likes to dilute things. • Water molecules pass easily through membranes. Osmosis words • HYPERTONIC – a solution has more solutes, or is more concentrated than another. • HYPOTONIC – a solution has fewer solutes, or is more dilute than another. • ISOTONIC – a solution has equal concentration to another. Smelly Balloons Like the plasma membrane, the balloon is a SEMIPERMEABLE MEMBRANE. Semipermeable means that the membrane allows movement of certain molecules across. Molecules small enough to fit through the pores in the balloon will move by DIFFUSION Both in and out of the balloon. The food extract smell molecules are small enough to diffuse through the membrane. Eventually, the diffusing molecules reach the smell receptors in your nose. Smelly Balloons • Each group will get 1 balloon initially. After 3 minutes you will rotate balloons. This will continue until all five balloons have been rotated. • Fill in balloon number, scent description, and strength of scent (none, weak, strong) in chart for all 5 balloons Balloon number Smell Description Smell Strength (None,Weak, Strong) 1 2 3 4 5 Questions 1. 2. 3. How do the smell molecules get out of the balloon? This process by which molecules move from a place of higher concentration (inside the balloon) to a place of lower concentration (outside the balloon) is called State two ways the latex skin of a balloon is like a cell membrane. A. B. 4. What is diffusion? 5. How do molecules move? 6. What is solute? 7. What is a solvent? 8. What is osmosis? 9. A. Which was the independent variable? B. Which was the dependant variable? C. Which was the control?