Modern Chemistry Chapter 12
advertisement

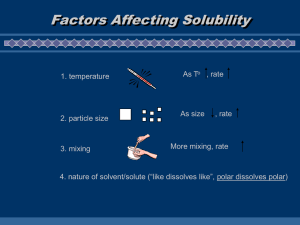
Modern Chemistry Chapter 12Solutions Section 1- Types of Mixtures • Solutions are homogeneous mixtures of two or more substances in a single phase. – Soluble describes a substance as capable of being dissolved. – Solvent is the dissolving medium in a solution. – Solute is the substance that is dissolved in a solution. Types of Solutions • Solutions can be in any of the three common physical states. solid- a mixture of metals called an alloy liquid- salt water, sugar water, KoolAid… gas- the atmosphere Suspensions & Colloids • A suspension has large particles that settle out of a solvent. eg. muddy water • A colloid has intermediate size particles. Also called an emulsion or a foam. Solutes: electrolytes vs. nonelectrolytes • An electrolyte is a substance that dissolves in water to give a solution that conducts electricity. • A nonelectrolyte is a substance that dissolves in water to give a solution that does NOT conduct electricity. • Do section review questions #1, #2, & #6 on page 406. Section 2- The Solution Process • Factors that affect the rate of solution (how quickly a substance dissolves): – Any process that increases the number of contacts between the solvent and the solute will increase the solution rate. • increasing the surface area of the solute • agitating (shaking or stirring) the solution • heating the solvent Solutions • Solution equilibrium is the physical state in which the opposing processes of dissolution and crystallization occur at equal rates. • A saturated solution contains the maximum amount of dissolved solute. • An unsaturated solution contains less than the maximum amount of dissolved solute. • A supersaturated solution contains more dissolved solute than a saturated solution. Solubility • Solubility is a measurement of how much solute will dissolve in a specific amount of solvent at a specific temperature to make a saturated solution. • “Likes dissolve likes”- polar solvents dissolve polar solutes & nonpolar solvents dissolve nonpolar solutes. • Hydrationis the solution process with water as the solvent. • Hydrates are ionic compounds that have formed crystals that have incorporated water molecules in their structure. • Immiscible liquids are not soluble in one another. eg. oil & water • Miscible liquids dissolve freely in one another in any proportion. eg. water & alcohol Solubility • Increasing the pressure has no effect on the solubility of a solid in a liquid but does increase the solubility of gases in a liquid. • Increasing temperature often increases the solubility of a solid in a liquid but decreases the solubility of a gas in a liquid. • Henry’s Law states that the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas on the surface of the liquid. • Effervescence is the rapid escape of a gas from a liquid in which the gas is dissolved. • A solvated solute particle is surrounded by solvent molecules. • Enthalpy of solution is the amount of heat absorbed by a solution when a specific amount of solute dissolves in a solvent. Problems • Do section review questions #1, #2, #3, #5, & #6 on page 416. • Read the CrossDisciplinary Connection on page 417 and answer the questions at the end of the reading. Section 3 Concentrations of Solutions • The concentration of a solution is a measure of the amount of solute dissolved in a given amount of solvent or solution. • Molarity (M) is the number of moles of solute in one liter of solution. M = #mol L • Do practice problems #1, #2, & #3 on page 421. Solution Concentrations • Molality (m) is the concentration of a solution expressed in moles of solute per kilogram of solvent. m = #mol kg Do practice problems #1 & #2 on page 424. Solution Concentrations • Percent composition by mass (%) is a concentration that expresses the percent of solute in a solution. % = #g solute x 100 # g solution Do section review problems #1, #2, & #3 on page 424. Chapter 12 Test Review • multiple choice (30) – – – – – – – – – – – define & identify suspensions & solutions define an alloy define & identify electrolytes & nonelectrolytes factors that affect the rate of dissolution definitions of unsaturated, saturated & supersaturated solutions general rules for predicting whether a solute is soluble in a solvent definition of solubility effects of temperature & pressure on the solubility of gases and solids in liquids definitions of molarity (M) and molality (m) solving molarity & molality problems FORMULAS: M = #mol/L m = #mol/kg