14.1
Properties of Gases
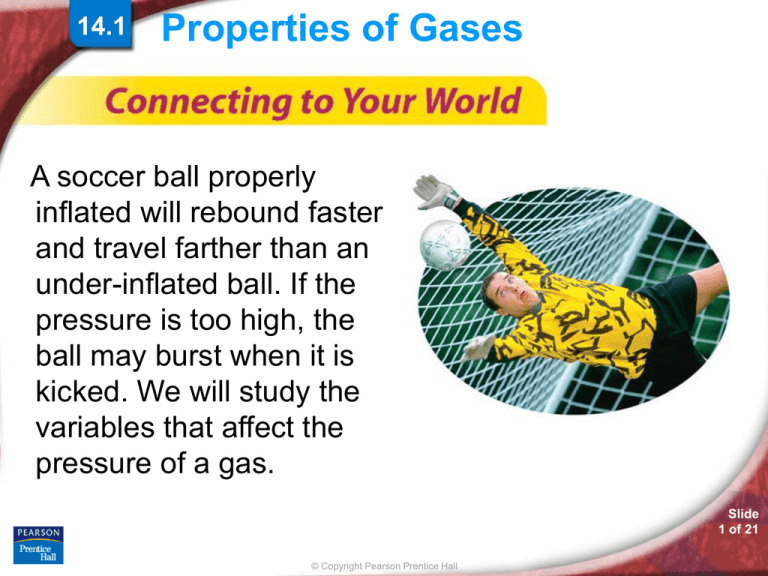
A soccer ball properly
inflated will rebound faster
and travel farther than an
under-inflated ball. If the
pressure is too high, the
ball may burst when it is
kicked. We will study the
variables that affect the
pressure of a gas.
Slide
1 of 21
© Copyright Pearson Prentice Hall
14.1
Properties of Gases >
Chemistry
Temperature
Matter
Rates of
Effusion and
Effusion
Pressure
Gas
Density
Volume
Moles
Molar Mass
Slide
2 of 21
© Copyright Pearson Prentice Hall
14.1
Properties of Gases > Compressibility
Compressibility
Why are gases easier to compress than
solids or liquids are?
Slide
3 of 21
© Copyright Pearson Prentice Hall
14.1
Properties of Gases > Compressibility
Compressibility is a measure of how much the
volume of matter decreases under pressure.
When a person collides with an inflated airbag,
the compression of the gas absorbs the energy
of the impact.
Slide
4 of 21
© Copyright Pearson Prentice Hall
14.1
Properties of Gases > Compressibility
Gases are easily compressed because of
the space between the particles in a gas.
• The distance between particles in a gas is
much greater than the distance between
particles in a liquid or solid.
• Under pressure, the particles in a gas are
forced closer together.
Slide
5 of 21
© Copyright Pearson Prentice Hall
14.1
Properties of Gases > Compressibility
At room temperature, the distance between
particles in an enclosed gas is about 10 times
the diameter of a particle.
Slide
6 of 21
© Copyright Pearson Prentice Hall
14.1
Properties of Gases > Factors Affecting Gas Pressure
Factors Affecting Gas Pressure
What are the three factors that affect gas
pressure?
•
Amount of Gas
•
Volume
•
Temperature
Slide
7 of 21
© Copyright Pearson Prentice Hall
14.1
Properties of Gases > Factors Affecting Gas Pressure
Four variables are generally used to describe a
gas. The variables and their common units are
• pressure (P) in kilopascals
• volume (V) in liters
• temperature (T) in kelvins
• the number of moles (n).
Slide
8 of 21
© Copyright Pearson Prentice Hall
14.1
Properties of Gases > Factors Affecting Gas Pressure
Amount of Gas
You can use kinetic theory to predict and explain
how gases will respond to a change of
conditions. If you inflate an air raft, for example,
the pressure inside the raft will increase.
Slide
9 of 21
© Copyright Pearson Prentice Hall
14.1
Properties of Gases >
Heat
Energy transferred due to differences in temperature
Temperature
Measure of the average kinetic energy of particles
composing a material
Pressure
Force per unit area
Volume
The amount of space a material occupies
Slide
10 of 21
© Copyright Pearson Prentice Hall
14.1
Properties of Gases > Factors Affecting Gas Pressure
Collisions of particles with the inside walls of the
raft result in the pressure that is exerted by the
enclosed gas.
If you increase the number of particles it will
increase the number of collisions, which is why
the gas pressure increases.
Slide
11 of 21
© Copyright Pearson Prentice Hall
14.1
Properties of Gases > Factors Affecting Gas Pressure
If the gas pressure increases until it exceeds the
strength of an enclosed, rigid container, the
container will burst.
Slide
12 of 21
© Copyright Pearson Prentice Hall
14.1
Properties of Gases > Factors Affecting Gas Pressure
Aerosol Spray Paint
Slide
13 of 21
© Copyright Pearson Prentice Hall
14.1
Properties of Gases > Factors Affecting Gas Pressure
Volume
You can raise the pressure exerted by a
contained gas by reducing its volume. The more
a gas is compressed, the greater is the pressure
that the gas exerts inside the container.
Slide
14 of 21
© Copyright Pearson Prentice Hall
14.1
Properties of Gases > Factors Affecting Gas Pressure
When the volume of the container is halved, the
pressure the gas exerts is doubled.
Slide
15 of 21
© Copyright Pearson Prentice Hall
14.1
Properties of Gases > Factors Affecting Gas Pressure
Temperature
An increase in the temperature of an enclosed
gas causes an increase in its pressure.
As a gas is heated, the average kinetic energy of
the particles in the gas increases. Faster-moving
particles strike the walls of their container with
more energy.
Slide
16 of 21
© Copyright Pearson Prentice Hall
14.1
Properties of Gases > Factors Affecting Gas Pressure
When the Kelvin temperature of the enclosed
gas doubles, the pressure of the enclosed gas
doubles.
Slide
17 of 21
© Copyright Pearson Prentice Hall
14.1 Section Quiz.
Assess students’ understanding
of the concepts in Section 14.1.
Continue to:
-or-
Launch:
Section Quiz
Slide
18 of 21
© Copyright Pearson Prentice Hall
14.1 Section Quiz.
1. Compared to liquids and solids, gases are
easily compressed because the particles in a
gas
a. attract each other.
b. are spaced relatively far apart.
c. are very small.
d. repel each other.
Slide
19 of 21
© Copyright Pearson Prentice Hall
14.1 Section Quiz.
2. Gas pressure is affected by
a. temperature, volume, and the amount of
the gas.
b. temperature, volume, and the molar mass
of the gas.
c. phase diagram, volume, and the size of the
container.
d. temperature, phase diagram, and the mass
Slide
of the gas container.
20 of 21
© Copyright Pearson Prentice Hall
14.1 Section Quiz.
3. For gases, the SI units for volume (V),
pressure (P), and temperature (T) are,
respectively,
a. liters, kilopascals, and °C.
b. liters, kilopascals, and kelvins.
c. cm3, kilopascals, and kelvins.
d. liters, atmospheres, and °C.
Slide
21 of 21
© Copyright Pearson Prentice Hall
The Gas Laws
This hot air balloon was
designed to carry a
passenger around the
world. You will study some
laws that will allow you to
predict gas behavior under
specific conditions, such
as in a hot air balloon.
Slide
22 of 21
© Copyright Pearson Prentice Hall
Boyle’s Law: Pressure and Volume
Boyle’s Law: Pressure and Volume
How are the pressure, volume, and temperature
of a gas related?
Slide
23 of 21
© Copyright Pearson Prentice Hall
Boyle’s Law: Pressure and Volume
If the temperature is constant, as the
pressure of a gas increases, the volume
decreases.
Slide
24 of 21
© Copyright Pearson Prentice Hall
Boyle’s Law: Pressure and Volume
a. Boyle’s law states that for a given mass of
gas at constant temperature, the volume of
the gas varies inversely with pressure.
Slide
25 of 21
© Copyright Pearson Prentice Hall
Boyle’s Law: Pressure and Volume
Slide
26 of 21
© Copyright Pearson Prentice Hall
Boyle’s Law: Pressure and Volume
Simulation 15
Examine the relationship between gas, volume
and pressure.
Slide
27 of 21
© Copyright Pearson Prentice Hall
14.1
Slide
28 of 21
© Copyright Pearson Prentice Hall
14.1
Slide
29 of 21
© Copyright Pearson Prentice Hall
14.1
Slide
30 of 21
© Copyright Pearson Prentice Hall
14.1
Slide
31 of 21
© Copyright Pearson Prentice Hall
for Sample Problem 14.1
Problem Solving 14.8
Solve Problem 8 with the
help of an interactive
guided tutorial.
© Copyright Pearson Prentice Hall
Slide
32 of 21






