Lipid composition Unsaturated
advertisement
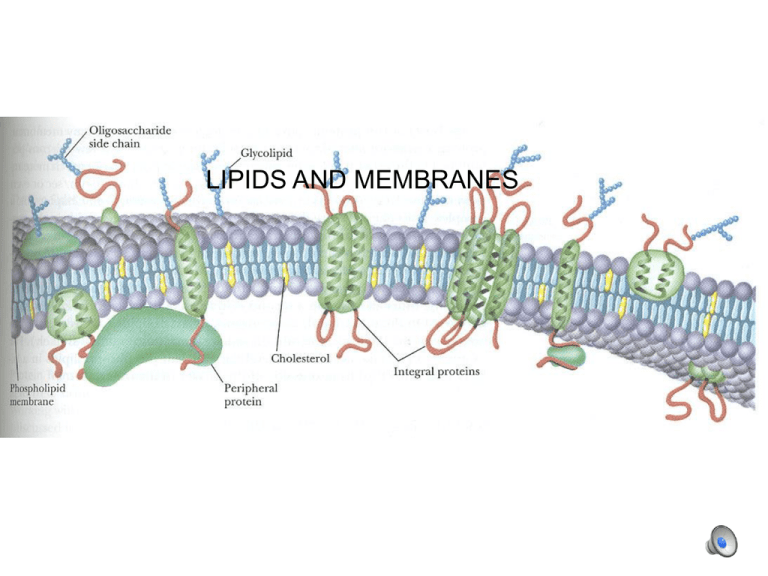
LIPIDS AND MEMBRANES Fatty acids H H C C H H •Hydrocarbon chain (saturated or un-) •Carboxylic acid group Fatty acids Nomenclature: C1 (COOH), C2, C3, etc. C = C2, ßC = C3, etc. 18 C’s with 2 double bonds: C18:2(9,12) (“9” means between C9 and C10) (double bonds are normally at 9, 12, 15 and are cis) • • • • Palmitic acid—C16:0 Stearic acid—C18:0 Oleic acid—C18:1 (9) Linoleic acid—C18:2 (9,12) Fatty acids • • • • Palmitic acid—16:0 Stearic acid—18:0 Oleic acid—18:1 (9) Linoleic acid—18:2 9,12) Long, straight chains are less soluble (in aqueous medium) Short chains, and double bonds reduce melting temperature and increase solubility Triacyl glycerol: energy storage (fats and oils): 38 kJ/mol (vs protein 17 kJ/mol) Fats and oils --storage forms of C and energy-- accumulate in lipid bodies An adipocyte Membrane lipids (phospholipids) glycerol C1-attached fatty acid normally saturated glycerol C2-attached fatty acid normally unsaturated glycerol C3: phosphate plus hydrophilic group... Membrane lipids (phospholipids): note the different head groups Other l ipids: e.g. sphingolipids on a sphingosine base Se e below: sphingosine is outl ined Membranes Lipid bilayer: heads in contact with aqueous solution; tails isolated from it. Note the different lipids in membranes: inner and outer leaflets are distinct. erythrocyte: inner: phosphatidylethanolamine, phosphatidylserine predominate outer: phosphatidyl choline, sphingomyelin predominate P-lipid breakdown by hydrolysis: catalyzed by phospholipases e.g.: snake venom P-lipase (PLA2) hydrolyzes C2 fatty acid, which bursts erythrocytes Sterols: note tetra-ring base, hydrophobic addition, hydrophillic -OH Archeal membrane lipids have structures analogous to phospholipids Lipid solubility on water surface: heads in water, tails in air submerged single tail lipids (e.g., sodium lauryl sulfate) at Òcritical micelle concentra tionÓ: spontaneous formation of micelles submerged phospholipids form liposomes, bilayer leaflets Phase transitions liquid crystal Lipid mobility phase transition heat absorption gel ToC T oC liquid crystal Lipid mobility phase transition heat absorption gel ToC T oC longer chains raise the transition temperature, decrease fluidity double bonds lower the transition temperature, increase fluidity membranes le ak during the tr ansition cholesterol (et al.) makes the gel more fluid and the liquid crystal l ess fluid (also Ca2+) enzymes in membranes generally work better in liquid crysta l phase, but co mplexes may stay together better in a gel Resistance to cold is associated with higher concentrations of linolenic acid. Olive oil “Tests indicate that imported “extra virgin”olive oil often fails international and USDA standards - UC Davis Olive Center, July 2010” Lipid composition Unsaturated Palmitic acid: 7.5–20.0% Stearic acid: 0.5–5.0% Mono-unsaturated Oleic acid: 55.0–83.0% Palmitoleic acid: 0.3–3.5% Polyunsaturated Linoleic acid: 3.5–21.0 % α-Linolenic acid: <1.5% Problems Free fatty acids Peroxides UV absorption (conjugated double bonds) 1,2 and 1,3 diacylglycerols Summary Fatty acids are distinguished by length and presence of double bonds: palmitic, steric, oleic, and linoleic acids are common. Storage lipids are generally triglycerides Membrane lipids include phospholipids, sphingolipids, and sterols Temperature-induced phase transitions represent a change from close-packed to more open conformations