File energy in earth processes
advertisement

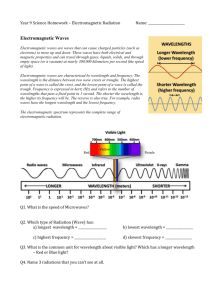
Energy in Earth Processes It’s All About Work Energy The ability to do work. Everything that is done in the universe involves the use or transfer of energy. ***Energy from the sun drives most Earth surface processes.*** Electromagnetic Energy Transverse waves radiated by the sun. Transverse waves vibrate at right angles to the direction of movement. Most familiar is visible light. ***Different types distinguished by wavelength.*** See page 14 of ESRT. Electromagnetic Spectrum Microwaves Electromagnetic Spectrum On the chart – Longer wavelength to the right, shorter wavelength to the left. ***Visible light is the only part that can be seen with the human eye.*** ***Heat is given off as infrared radiation.*** What happens when electromagnetic energy comes in contact with a material? It can be refracted or bent. The direction of the waves are changed. It can be reflected or bounced off. Transmitted or passed through. Absorbed or taken into the material. – Dark colors absorb more than light colors. – Rough texture absorbs more than smooth. – ***Thus, dark, rough objects are the best absorbers of the sun’s energy.*** Energy Transfer Energy (heat) moves from an area of high concentration (source) to an area of low concentration (sink). This will continue until the energy between the source and the sink are even (dynamic equilibrium). 3 Methods of Transfer Conduction, convection and radiation Conduction – transfer of heat energy from atom to atom or molecule to molecule. Ex. Hot pot handle. Convection – transfer of heat by movement in fluids, liquids and gasses. Convection currents transfer heat throughout Earth’s atmosphere, hydrosphere and mantle. Ex. Smoke rising from a campfire. Radiation – heat transferred via electromagnetic waves. Can go across empty space. Fastest – travels at the speed of light. Heat & Temperature Temperature – average kinetic energy of particles in a substance. Heat – total kinetic energy of particles in a substance. Heat always flows from higher energy to lower energy. Calorie Different substances heat up at different rates. Unit for measuring heat quantity is the calorie. Defined as the quantity of heat needed to raise the temperature of 1 gram of water 1 degree Celsius. Specific Heat The quantity of heat required to raise the temperature of 1 gram of a substance 1 degree Celsius. Every substance has its own specific heat, and it is different from all other substances. Liquid water is the highest at 1.0 cal/goC. Page 1 ESRT. In simple terms specific heat is the resistance of a substance to heating up or cooling off. ***Large bodies of water have a major moderating effect on weather and climate because water has the highest specific heat of any natural substance.*** Heat Energy and Changes of State Melting – solid to liquid Solidification or freezing – liquid to solid Evaporation or vaporization – liquid to gas Condensation – gas to liquid Sublimation – solid to gas Stored Heat and Changes of State When a material is in one of the 3 states, its temperature rises as heat is added to it. If the material is in the process of changing state, its temperature remains the same.