Section 5.4
advertisement

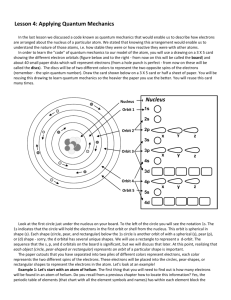
Science Academic 10 - Chemistry Atoms – Bohr Rutherford Model Science Perspectives – Section 5.4 Pages 184-187 Bohr-Rutherford Model of the Atom Electrons move in orbits around the nucleus Electrons do NOT exist between orbits. However, electrons can move between orbits. There are a maximum number of electrons in each orbit. Orbit 1 can have two electrons Orbit 2 can have eight electrons Orbit 3 fills until it has eight electrons. Then, Orbit 4 starts adding electrons until it has two electrons. Then, Orbit 3 begins filling again until it has a maximum of 18 electrons. Orbit 4 can have 32 electrons (Na). Sodium has an Atomic Number of 11. Sodium has 11 protons in the nucleus and 11 electrons in its orbits. The 11th electron is found in Orbit #3. HOMEWORK Page 187 – Questions 4, 8, 9