lesson 10 aromatics - Keith Grammar School
advertisement

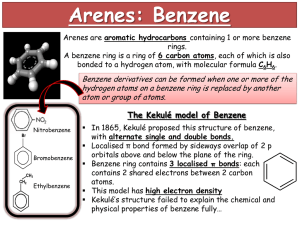
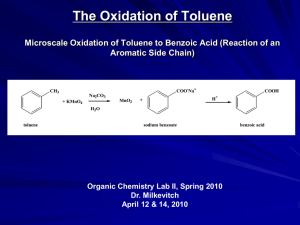
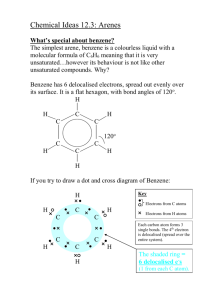
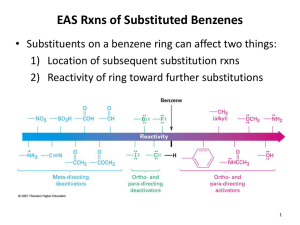
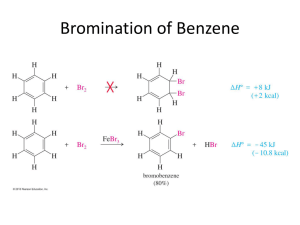
Advanced Higher Chemistry Unit 3 Aromatics Aromatics Aromatics are hydrocarbons containing the benzene ring (C6H6). The systematic name for the family of alkyl substituted aromatic hydrocarbons is the arenes. Bonding in Benzene Structure (a) was the first sturcture proposed by Kekule in 1865 however this structure was proposed incorrect with x-ray diffraction results and the fact that benzene is resistant to addition reactions. (b) and (c) are resonance structures and differ only in the arrangement of the electrons but do not actually exist . The real structure of benzene is best represented by (d) The six carbons in benzene are sp2 hybridised. The carbons are bonded to each other and the hydrogen atoms by sigma bonds. Each carbon has a p orbital containing a single electron that can overlap with neighbouring orbitals to form pi bonds. This area of negative charge is above and below the ring (delocalised p electron system). The six electrons become delocalised and occupy two continuous doughnutshaped electron clouds above and below the planar sigma framework. The delocalisation of the electrons help to bond the atoms more tightly together. The result is a completely symmetrical molecule, with considerable stability. The term ‘aromatic’ can be defined to describe any system that contains a ring of atoms stabilised by delocalised pi electrons. Molecules with straight or branched chains are described as aliphatic. Reactions of benzene Benzene tends to undergo electrophilic substitution reactions. The areas of high electron density attract positive reagents. The electrophile attacks one of the carbon atoms forming an intermediate ion with a positive charge. The intermediate ion then loses a hydrogen ion. The aromatic nature of the hydrocarbon is then restored. Reactions with bromine or chlorine Benzene can undergo electrophilic substitution by bromine in the presence of a catalyst (e.g. iron (III) bromide, iron (III) chloride, aluminium (III) chloride). There are parallels in the catalysed reaction of bromine with benzene with the reaction of bromine to an alkene. Since benzene is less reactive than alkenes, the catalyst is needed to polarise the bromine molecule creating an electrophilic centre. The partially positive bromine atom can then attack the benzene ring. Heterolytic fission of the Br-Br bond occurs to form a carbocation, just like the bromination of an alkene. However, the similarity ends there and, by losing a hydrogen ion in the final step, the aromatic system is restored and the catalyst regenerated. The overall effect is that one of the hydrogen atoms of the benzene molecule has been replaced by a bromine atom i.e. an electrophilic substitution reaction has taken place. In the dark, chlorine undergoes a similar reaction with benzene to form chlorobenzene. In the light, chlorine adds to benzene by a different mechanism to form 1,2,3,4,5,6hexachlorocyclohexane (one of the very few addition reactions of the benzene ring). Nitration of benzene Benzene reacts with a mixture of conc.nitric acid and conc. sulphuric acid to from nitrobenzene. This reaction is of great importance to industry since the nitrobenzene can be reduced to produce phenylamine, C6H5NH2, also known as aniline, which is an important intermediate in the manufacture of dyes. The mechanism for nitration involves electrophilic attack by the very reactive nitronium cation NO2+, which is produced when conc. nitric acid reacts with conc. sulphuric acid The nitronium cation is a powerful electrophile and attacks the benzene molecule as shown. Further substitution can occur to produce di- and tri- compounds. Further substitution can occur to produce di- and tri- compounds. Sulphonation of benzene Benzenesulphonic acid can be produced by electrophilic substitution if benzene is heated with conc. sulphuric acid under reflux. The same product is formed in the cold when using fuming sulphuric acid, which is a solution of sulphur trioxide in conc. sulphuric acid. The electrophile in sulphonation is believed to be the SO3 molecule, either free or in combination with acid, which helps to turn it into a strong enough electrophile to attack the benzene molecule. The SO3 molecule is electron deficient and carries a partial positive charge on the sulphur atom. The mechanism is similar to that for nitration. This reaction is important in the production of synthetic detergents. Alkylation of benzene : Friedel-Crafts Reaction As for the reaction of benzene with bromine, a halogenalkane is made polar by the action of a catalyst (e.g. aluminium (III) chloride). The C-Cl bond is already polar. The catalyst increases the polarity and may even cause the bond to break heterolytically to form a carbocation. In either case, the power of the electrophile is increased to allow it to attack the benzene ring to form a monoalkylbenzene This reaction is used industrially in the manufacture of synthetic dyes, the production of phenol and propanone, and in the manufacture of nylon. Although electrophilic substitution is the typical reaction of the benzene ring, the presence of substituents on the ring has an influence on the outcome of this reaction. Some substituents increase the susceptibility to electrophilic attack while others decrease it. On the other hand, the benzene ring also has an influence on the behaviour of such substituents. In the next slides phenol and phenylamine (aniline) will be considered. Phenol Phenols are aromatic compounds with a hydroxyl group attached to the benzene ring. The OH bond is polar and can be broken heterolytically to produce H+ ions, under certain circumstances. The OH group is therefore potentially acidic. Phenol has a higher Ka value than ethanol therefore is more acidic. When phenol acts as an acid, it produces the phenoxide ion in which the negative charge on the oxygen atom can be partly delocalised into the aromatic pi system. This delocalisation can be illustrated using possible resonance structures for the phenoxide ion. The resonance structures show how the negative charge on the oxygen atom is decreased, thus stabilising the phenoxide ion and making it less likely to accept a proton. This effect is confirmed by phenol’s Ka value lying between an alcohol and an alkanoic acid i.e. phenol is more acidic than an alcohol but less acidic than an alkanoic acid. Phenylamine Phenylamine (aniline) is an aromatic, basic amine. The lone pair of electrons on the nitrogen atom can accept a hydrogen ion and form the phenylammonium ion. Phenylamine is a weak base as the Ka of the resulting conjugate acid is high. Base Conjugate acid Ka at 25oC ammonia Ammonium ion 5.5. x 10-10 ethylamine Ethylammonium ion phenylamine Phenylammonium ion 1.9 x 10-11 2 x 10-3 Phenylamine is less basic than both ammonia and ethylamine. The decrease in basicity can be explained by looking at its resonance structures. The lone pair of electrons on the nitrogen atom are delocalised into the aromatic pi system therefore the nitrogen atom is less able to act as a base. Such delocalisation stabilises the phenylamine ion in a way that is not possible for the phenylammoniumion. Exercise Now try the exercise on page 57 of your Unit 3(b) notes.