Synthetic Polymers

Synthetic Polymers
Synthetic Polymers
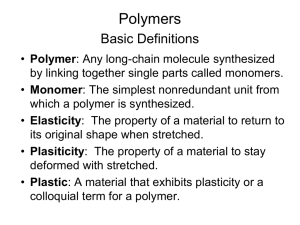
A polymer is a substance composed of macromolecules, that have many of atoms and a very high molecular weight.
Starch, cellulose, silk, and DNA are naturally occurring polymers.
Synthetic polymers include nylon, polyethylene, polystyrene and Bakelite, among countless others.
The reactions used to prepare polymers are the same fundamental reactions of simple organic compounds.
27.1
Some Background
Some Background
In 1839 Goodyear modified natural rubber: vulcanization.
Synthetic fibers called rayons made by chemical modification of cellulose at the end of the 19 th century.
Bakeland first synthetic polymer patented in
1910. Made from phenol and formaldehyde.
In the 1920’s it became known that polymers were high molecular weight materials held together by covalent bonds.
27.2
Polymer Nomenclature
Polymer Nomenclature
Source based naming most accepted. Name polymers according to the monomer from which they are made.
Acrylonitrile Polyacrylonitrile Vinyl chloride Poly(vinyl chloride)
Convention for drawing polymer formulas is to enclose the repeating unit within brackets and use the subscript n to denote the large number of repeating units.
Polymer Nomenclature
Both poly(ethylene glycol) and poly(ethylene oxide) are made from ethylene oxide and have the same repeating unit.
Some polymers are simply called by their trade name for example polytetrafluoroethylene is always called Teflon.
27.3
Classification of Polymers:
Reaction Type
Addition Polymers
Addition polymers are formed by reactions of the type
A + B A-B where A-B retains all the atoms of A and B. When A = B this is a homopolymer .
Polystyrene is a homopolymer.
Styrene Polystyrene
Addition Polymers
A + B A-B
When A and B are different it is a copolymer .
Saran is a copolymer of vinyl chloride and vinylidene chloride.
Vinylidene chloride
Vinyl chloride
Saran
Addition Polymers
Polymers prepared from alkenes are called polyolefins .
Formaldehyde polymerizes to form polyformaldehyde.
Heating polyformaldehyde reforms formaldehyde.
Formaldehyde Polyformaldehyde
Condensation Polymers
Condensation polymers are prepared by covalent bond formation with loss of a small molecule like water, an alcohol or a hydrogen halide.
A simple condensation reaction gives a single product.
With two difunctional molecules the product of the first reaction continues to react.
Condensation Polymers: Polyamides
Most familiar condensation polymers are polyesters, polyamides and polycarbonates.
Aramids are polyamides formed from aromatic components and are string and stiff polymers. Kevlar used in bullet proof vests and helmets is shown.
1,4-Benzenediamine
Terephthaloyl chloride
Kevlar
27.4
Classification of Polymers:
Chain Growth and Step Growth
Chain Growth Polymers
In the chain growth process monomers add one-by-one to one end of the growing chain. There is only one growth point.
The concentration of monomer decreases steadily until it is consumed and chain growth stops.
Step Growth Polymers
In the step growth process monomers form a series of oligomers that react together to form the polymer.
The concentration of monomer decreases early forming the oligomers which continue to react.
27.5
Classification of Polymers:
Structure
Linear Polymers
Linear polymers have a continuous chain. The collection of chains could be random (amorphous) to highly ordered
(crystalline).
Crystallites
Crystalline domains are called crystallites.
Degree of crystallinity is the percentage of crystallites.
Branched Polymers
Branched polymers have branches extending off the main chain.
Branching decreases both crystallinity and density and results in a softer less rigid polymer, eg LDPE.
Crosslinked Polymers
Crosslinked or network polymers have linking connections between chains.
Vulcanization uses sulfur to crosslink the chains in rubber.
Classification of Polymers: Recycling
27.6
Classification of Polymers:
Properties
Thermoplastic Polymers
Thermoplastic polymers soften when heated.
At the glass transition temperature they change from glass to a rubbery state.
Crystalline polymers also melt at the melting temperature.
Thermosetting Polymers
Thermosetting polymers pass through a liquid phase and then solidify (cure) on continued heating. The solid material has covalent bonds linking strands.
Melamine and Bakelite are examples.
Elastomers
Elastomers are flexible polymers that can be stretched but return to their original shape when the force is released.
27.7
Addition Polymers:
A Review and a Preview
Free-Radical Polymerization
Polymerization of alkenes.
Radical initiators.
Free-Radical Polymerization
Chain termination steps.
Combination.
Disproportionation.
Coordination Polymerization
Coordination polymerization catalysts are usually transition metal complexes.
Bis(cyclopentadienyl)zirconium dichloride
Common terminating reaction is elimination.
27.8
Chain Branching in
Free-Radical Polymerization
Chain Branching in Free-Radical Polymerization
Propagation step.
Branches must be formed by other reactions.
1. Intramolecular hydrogen atom abstraction.
2. Intermolecular hydrogen atom abstraction or chain transfer.
Mechanism of Intramolecular H-atom Transfer
The reaction.
Step 1. 1,5-H-atom abstraction.
=
Mechanism of Intramolecular H atom Transfer
Step 2. Radical Addition.
Step 3. Multiple successive radical additions.
Mechanism of Intermolecular H-atom Transfer
Step 1. H-Atom abstraction between chains.
Step 2. Addition.
27.9
Anionic Polymerization:
Living Polymers
Anionic Polymerization of Styrene
Reaction equation.
Addition of butyl lithium to styrene is rapid and many initiation sites simultaneously formed resulting in a narrower range of molecular weights.
Mechanism of Anionic Polymerization
Step 1. Nucleophilic Addition.
Step 2. Nucleophilic Addition.
Mechanism of Anionic Polymerization
Step 3. Continued addition.
Final product can be:
(a) protonated by water to form polystyrene; or
(b) reacted with a second monomer.
Living Polymers
The final product of the anionic polymerization is an anion which can still react so it is called a living polymer .
Monomers for Anionic Polymerization
Monomers with electron withdrawing substituents are especially susceptible to anionic polymerization.
Acrylonitrile Methyl acrylate Methyl 2-cyanoacrylate
Methyl 2-cyanoacrylate is used in Super Glue and it is so active that polymerization can be initiated by weak bases such as atmospheric moisture.
27.10
Cationic Polymerization
Cationic Polymerization
Best monomers are those that form relatively stable cations like 2-methylpropene which forms the polymer known as polyisobutylene.
One initiator used is boron trifluoride with a trace of water.
Mechanism of Cationic Polymerization
Reaction equation.
Step 1. Protonation.
Step 2. Addition.
Mechanism of Cationic Polymerization
Step 3. Repetitions of Step 2.
Step 4. Deprotonation.
27.11
Polyamides
Nylon Synthesis
Nylon 66 is prepared from a 6-carbon dicarboxylic acid and a 6-carbon diamine. The acid –base reaction gives a salt that is heated to form the polymer.
Nylon 66
Hydrogen Bonding and Polyamides
Nylon 66 resembles silk in both structure and properties.
Nylon 6
Nylon 6 is formed by heating 6-aminohexanoic acid.
27.12
Polyesters
Polyesters
Best known polyester is poly(ethylene terephthalate), PETE, prepared from ethylene glycol and terephthalic acid.
Benzene-1,4-dicarboxylic acid terephthalic acid
Poly(ethylene terephthalate)
Polyesters
Reaction with benzene-1,2-dicarboxylic acid and glycerol
(1,2,3-propanetriol) forms the alkyd resins used in paint.
Polyesters
Surgical sutures made from poly(glycolic acid) and poly(lactic acid) are durable but slowly dissolve.
27.13
Polycarbonates
Polycarbonates
Polycarbonates are polyesters of carbonic acid.
Disodium salt of bisphenol A
Phosgene
Bisphenol A polycarbonate (Lexan)
Lexan is a clear, transparent, strong, and impact-resistant plastic. It is used in both protective and everyday eyeglasses. The
Apollo 11 astronauts wore Lexan helmets.
27.14
Polyurethanes
Polyurethanes
Urethanes, or carbamates, contain this functional group.
Prepared from alcohols and isocyanates.
Isocyanate Urethane
For polymer synthesis need a diol and a diisocyanate.
A major use of polyurethanes is in spandex fibers. Other applications include in paints, adhesives, and foams.
Polyurethanes
Polyurethane synthesis generally uses a polymeric diol.
The diisocyanate is a mixture of 2,6- and 2,4-diisocyante.
27.15
Copolymers
Copolymers
Copolymers are classified according to the distribution of monomers in the macromolecule.
1. Random
2. Block
3. Graft
Random Copolymers
There is no pattern to the distribution of monomer units in a random copolymer.
Styrene –butadiene rubber (SBR) for automobile tires is a random copolymer.
Block Copolymers
The polymer has sections (blocks) of repeating units from different monomer units.
The living polymers generated by anionic polymerization are well suited to the preparation of block polymers.
Block Copolymers
The living polystyrene polymer reacts with butadiene to form a block of polybutadiene.
Graft Copolymers
The main chain bears branches (grafts) that are derived from a different monomer.
A graft copolymer of styrene and 1,3-butadiene is called
“high-impact polystyrene” and is used in laptop cases.
Synthesis of Graft Copolymers
Styrene is polymerized in the presence of polybutadiene.
Step 1. Allylic H-atom abstraction.
Synthesis of Graft Copolymers
Step 2. Addition.
Synthesis of Graft Copolymers
Step 3. Polystyrene reacts to form a “graft”.
Conducting Polymers
Henry Letheby formed a partially conducting material in 1862 by anodic oxidation of aniline in sulfuric acid. The material was a form of polyaniline now a widely used conducting polymer.
Conducting Polymers
The conducting polymer used in organic light-emitting diodes
(OLEDs) used for flat panel displays in TVs and cellphones.