File
advertisement

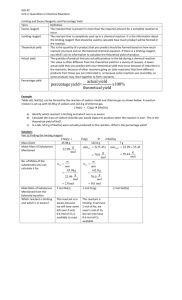
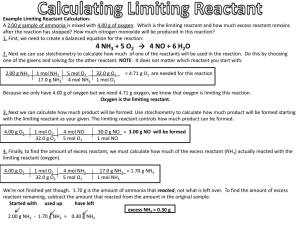
STOICHIOMETRY Stoichiometry is the science of using balanced chemical equations to determine exact amounts of chemicals needed or produced in a chemical reaction. Determining Ratios in Balanced Chemical Reactions What is the balanced chemical reaction for the reaction between nitrogen gas (N2) and hydrogen gas (H2) to produce ammonia (NH3)? This means that for every one mole of N2 you have (28.02 g) you need to have 3 mole of H2 (3 × 2.02 g = 6.06 g)… (34.08 g of reactants) You make 2 mole of NH3 ( 14.01 + 3.03 = 17.04 * 2 = 34.08 g) Mole Ratios These are what we call mole ratios Example 1: If you had 5 mol of N2, how many mol of H2 would you need? How many mol of NH3 would you make? Example Problem 2: Calculations Involving Mass of Reactants Propane, C3H8(g), is a gas that is commonly used in barbeques. Calculate the mass of oxygen gas, O2, that is needed to burn 15 g of propane. (Produces CO2 and H2O) Example Problem 2: Calculations involving Numbers of Entities and Mass How many molecules of oxygen are produced from the decomposition of 12 g of water into its elements? Recall: O2 and H2 are produced Practice Problems: 1. Bauxite ore contains aluminum oxide, Al2O3, which is decomposed using electricity to produce aluminum metal and oxygen gas (O2). What masse of aluminum metal can be produced from 125 g of aluminum oxide? How many grams of O2 are produced? 2. Potassium metal, K(s), reacts with hydrochloric acid, HCl(aq), to produce aqueous potassium chloride and hydrogen gas, H2. How many grams of potassium are required to produce 5.00 g of hydrogen gas? 3. Potassium chlorate, KClO3, decomposes when heated to form solid potassium chloride and oxygen gas. How many grams of KClO3 must decompose to produce 0.96 g of O2? Limiting Reactant, Excess Reactant and Percent Yield In the real world, you never have perfect amounts of every reactant you need… Example In the synthesis of water, we require exactly 2 mol of H2 and exactly 1 mol O2 to create exactly 2 mol of H2O… What would happen if we have a 2 mol H2 and 8 mol of O2? Would this change the amount f H2O that would be created? No… There O2 would be 7mol of O2 left over. is an excess reagent; H2 is limiting Balanced Equation 2 H2 O2 2H2O Moles 2 1 2 Molar Mass 2.02 g/mol 32.00 g/mol 18.02 g/mol Mass 4.04 g 32.00 g 36.04 g Balanced Equation 2 H2 O2 2H2O Moles 2 8 2 Molar Mass 2.02 g/mol 32.00 g/mol 18.02 g/mol Mass (Needed) 4.04 g 32.00 g Mass (Used Up/Made) 4.04 g 32.00 g Left Over 0.00 g 224.00 g 36.04 A chemical reaction will stop once any one of the reactants runs out. This reactant is known as the limiting reagent (limiting reactant) Any other reactant is an excess reagent Example 1: Table Salt, BaCL, can be formed by the reaction of sodium metal and chlorine gas. A reaction mixture contains 45.9 g of sodium and 142.0 g of chlorine. Calculate the mass of sodium chloride that is produced. Example 2: Determine the mass of carbon monoxide that is produced when 32.1 g of methane, CH4 undergoes incomplete combustion with 160.0 g of oxygen (products: CO and H2O) Example 3: Phosphorus, P4, reacts with chlorine gas to produce solid phosphorus pentachloride as the only product. Determine the mas of phosphorus pentachloride that is produced from a reaction between 123.88 g of phosphorus and 950.00 g of chlorine. Percentage Yield Yield: the quantity of product produced in a chemical reaction Actual Yield: the quantity of product that is actually produced in a chemical reaction Theoretical Yield: the quantity of product calculated from a balanced chemical equation (using stoichiometry) There are many reasons why the amount of a product predicted for a reaction may not actually form Reactions may not go to completion Some of the reactant may be impure There may be competing side reactions It may be difficult to collect the product Example 1: Iron is produced from its ore, hematite, Fe2O3, by heating hematite with carbon monoxide in a blast furnace. If 635 g of iron is obtained from 1150 g of hematite, what is the percentage yield of iron? Practice 1: The most common ore of arsenic, FeSAs, can be heated to produce arsenic and iron(II) sulfide. When 250 g of the ore was processed industrially, 95.3 kg of arsenic was obtained. Calculate the yield of arsenic. Homework… You have a test on Monday, November 24 at 6:30 pm, sharp. I strongly recommend you study for it.