Chapter 27 - Cells and Batteries
advertisement

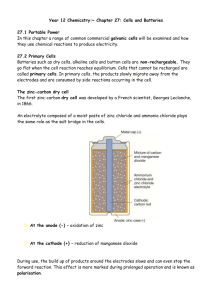
Chapter 27 – Cells and Batteries Primary Cells • Batteries such as dry cells, alkaline cells and button cells have one common feature; they are non-rechargeable. • Cells that cannot be recharged are called primary cells. • In primary cells, the products slowly migrate away from the electrodes and are consumed by side reactions occurring in the cells. Zinc-Carbon Dry Cell • This was the first small scale source of electrical energy. • An electrolyte composed of a moist paste of zinc chloride and ammonium chloride and it plays the same role as the salt bridge. • At the anode (-), oxidation of the zinc case produces the electrons. • At the cathode (+), reduction of manganese dioxide. Zinc-Carbon Dry Cell cont… • A new cell produces about 1.5 volts, but this diminishes during use. • To maintain a net forward reaction, the soluble reaction products must migrate away from the electrodes. • During use, the build up of products at the electrodes slows and can stop the forward reaction. • This is known as polarisation. • If a cell is allowed to rest, some of the products migrate away from the electrodes. • However, once the cell reaches equilibrium, the cell will be flat. Alkaline Cells • Have largely replaced Zinc-Carbon Cells. • The chemical reaction within an alkaline cell is similar to the Zinc-Carbon cell but the construction is totally different. • The alkaline cell is optimised for performance and longevity. • At the anode, zinc powder around the central metal rod is oxidised and once the ion is formed it reacts immediately with the OH- ions in the electrolyte to form zinc hydroxide. • At the cathode, manganese dioxide is reduced. • The improvements in this cell give it about five times the life of the Zinc-Carbon cell. Button Cells • Are used in very small devices. • There are two main types: silver-zinc cells and lithium cells. • Lithium button cells produce about 3 volts during discharge and silver-zinc give an almost constant 1.6 volts. Rechargeable Cells and Batteries • Rechargeable cells are known as secondary cells or accumulators. • To recharge a cell, the products of the reaction must be converted back into the original reactants. • This is done by connecting the cell to a charger, a source of electrical energy, which has a potential difference greater than the potential difference of the cell. • Electrical energy supplied is converted into chemical energy in the cell. • In order for it to be possible to regenerate the reactants, the products formed in the cell during discharge must remain in contact with the electrodes in a convertible form. Car Batteries • Lead-acid batteries are the most widely used secondary cells. • This is a car battery. • Although they appear to be a single unit, they are actually six separate cell connected together in a series. • Each cell contains three positive electrodes sandwiched between four negative electrodes. Car Batteries cont… • The electrodes are separated by a porous separator. • The positive electrodes consist of a lead grid packed with lead oxide. • Negative electrodes consist of a lead grid packed with powdered lead. • A solution of sulfuric acid acts as the electrolyte. • Each cell has a potential difference of just over 2 volts, a car battery has six of these so the total potential difference is about 12volts. Car Batteries cont… • At the anode: – Pb(s) + SO42-(aq) PbSO4(s) + 2e- • At the cathode: – PbO2(s) + SO42-(aq) + 4H+(aq) + 2e- PbSO4(s)+ 2H2O(l) • The overall equation: – Pb(s) + PbO2(s) + 2SO42-(aq) + 4H+(aq) 2PbSO4(s) + 2H2O(l) The product of both electrode reactions forms a solid on the surface of the electrodes, enabling the battery to be recharged. Car Batteries cont… • To recharge the battery, the electrode reactions are reversed. • The alternator is used to force electrons into the battery’s negative terminal and draw them out at the positive terminal. Nickel-based Cells and Batteries • The nickel based cells consist of a coiled anode, porous separator and cathode immersed in a concentrated KOH electrolyte. • At the anode: – For nickel-cadmium cells: Cd(s) + 2OH-(aq) Cd(OH)2(s) + 2e– For nickel-metal hydride cells: MH(s) + OH-(aq) M(s) + H2O(l) + e- • At the cathode: – NiOOH(s) + H2O(l) + e- Ni(OH)2(s) + OH-(aq) Nickel-based Cells and Batteries • The electrode reactions are fully reversible. • This enables the reactants to be regenerated when the cell is recharged. • Most brands of nickel-cadmium cells are capable of 1000 discharge-recharge cycles. • Nickel-metal hydride cells will do around 500 cycles. Fuel Cells • Cells can be constructed in which the reactants are supplied continuously allowing constant production of electrical energy. • These devices are called fuel cells. • Fuel cells transform chemical energy directly into electrical energy. • At the anode: – H2(g) + 2OH-(aq) 2H2O(l) + 2e- • At the cathode: – O2(g) + 2H2O(l) + 4e- 4OH-(aq) • The overall reaction: – 2H2(g) + O2(g) 2H2O(l) Fuel Cells cont… • Each cell produces about one volt. • Higher voltages are obtained by connecting a number of fuel cells in series. • The only by-products are water and heat. • The has been development of an acid fuel cell – this cell uses concentrated phosphoric acid as the electrolyte and air as the source of oxygen. It uses hydrogen but does not require it to be pure. Fuel Cells cont… • The use of fuel cells in transportation improves fuel efficiency and reduces greenhouse gas and other emissions. • Stationary fuel cell systems are used to generate electricity for domestic, commercial and industrial purposes. • A methanol powered fuel cell has been developed for use with portable electronic equipment such as mobile phones. Advantages of Fuel Cells • Convert chemical energy directly to electrical energy, better efficiency. • Water is a by-product, no greenhouse gases released. • Will generate electricity for as long as fuel is supplied, in conventional batteries electricity production stops once the reaction reaches equilibrium. • Use a variety of different fuels. • Electricity can be generated on site. Disadvantages of Fuel Cells • Require a constant fuel supply. • They are expensive. • Some types of fuel cell use expensive electrolytes and catalysts. • Fuel cells generate a direct current (DC), electrical appliances used in homes and industry rely on alternating current (AC). • The effectiveness of some fuels cells are affected by impurities in the hydrogen fuel. • Use of fuel cells in transport is limited by lack of facilities for hydrogen storage and distribution.