Document
advertisement

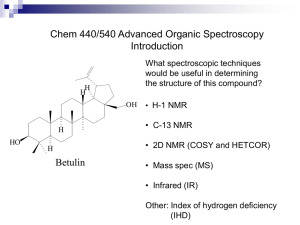
Wed. evening (Mar. 28) Expt.21; finish 16 Thurs. afternoon (Mar. 29) Expt. 21; finish 16 Fri. afternoon (Mar. 30) Expt. 21; finish 16 Mon. afternoon (Apr. 2) Expt. 21; finish 16 Mon. evening (Apr. 2) Expt. 21; finish 16 This reaction is regioselective (produces one regioisomer preferentially over one or more other possible regioisomers). In most cases, the thermodynamically most stable isomer is the major product in such reactions. Menthyl chloride This reaction is an example of the E2 mechanism. Zaitzev’s Rule is followed in this case. Neomenthyl chloride reacts much more rapidly than menthyl chloride. What do these results tell us about the reaction mechanism? exists in two chair conformers with one being much more stable than the other. The chloro group is anti-periplanar to a hydrogen (a requirement for the E2 mechanism) only in the minor conformer. Because only the minor conformer reacts, the reaction rate is quite low and leads to the formation of only one product. On the other hand, the major conformer (but not the minor) of neomenthyl chloride can react by the E2 mechanism. It can eliminate via two different pathways. OEt Zaitzev’s Rule is followed since it leads to the more stable alkene product. In 1994, Prof. David Todd (then a chemistry professor at Pomona College) was distilling a mixture of 2-methylcyclohexanol and acid to obtain the alkene products when he was invited to lunch by the dept. secretary. He stopped the distillation and went to lunch. When he returned, he switched receiving flasks before resuming the distillation. After working up both fractions, he was surprised to discover the second fraction contained much less of the expected major product, 1methylcyclohexene than the first. Prof. Todd named this unexpected result the “Evelyn Effect” after the dept. secretary. + H2O Up until this point, this reaction had been considered to be a good example of Zaitzev’s Rule and the E1 mechanism in alcohol dehydration reactions. Closer examination reveals that when 2methylcyclohexanol and phosphoric acid is distilled, the first 10% of the distillate is ~93% A, while the last 10% is only ~55% with most of the remainder being B, along with a trace of C. Previous researchers had reported that cis-2methylcyclohexanol undergoes dehydration much more rapidly than the trans isomer. minor amt B mainly A B Trans-2-methylcyclohexanol can react by an E2 mechanism only through its minor chair conformer, which leads only to product B. 4-methylcyclohexanol would be expected to produce 4-methylcyclohexene (D) with either the E2 or E1 mechanisms. If 4-methylcyclohexanol reacts via an E1 mechanism, are there any other alkene products that would be expected? A series of 1,2-hydride shifts can lead to other carbocation intermediates. In addition to D, it is possible to form alkenes A and B and possibly, a trace amount of C. If A and B form from 4methylcyclohexanol, what does that tell you about the reaction mechanism? If both the E2 and E1 mechanisms are operating in this reaction, can we say something about the relative magnitude of the rate constants, k 1 and k2? Reaction rate = k1[ROH] + k2[ROH][H3PO4] Working with a partner, your job will be to verify the existence of the Evelyn Effect for the dehydration of 2-methylcyclohexanol (mixture of cis and trans isomers) and to see if a similar effect occurs with 4methylcyclohexanol (mixture of cis and trans isomers). Propose a mechanistic hypothesis to explain your results and speculate about some possible causes of the Evelyn Effect. The lower boiling points of the alkene products relative to the alcohols, makes it possible to distill over an alkene/water mixture as they form. The still-head temperature during the distillation will be lower than the bp of the pure alkenes. Collect about 8 mL in a graduated cylinder for the first fraction and about 6 mL for the second fraction. Watch the still-head temperature carefully and stop the distillation when it starts to drop or the liquid in your distilling flask is low. If the liquid completely distills and you see foaming and white fumes, remove the heat source from your distilling flask before it forms a black tar that is difficult to remove from the flask. Chem 309 - Exp 21 As you know, much of each grade for th e experiments in this course depends on the quality of your discussion sections, which should always be clear and concise. The discussion section for Exp 21 is the most challenging of the semester, and will count for 30 out of the 55 points. Refer to the book, and your lecture notes for detailed mechanisms, and remember the following: · You will work in pairs: each individual will perform one acid-catalyzed dehydration reaction. One member of each pair will use 2methylcyclohexanol as the starting compound, and the other will use 4methylcyclohexanol. Each pair must then analyze the results of BOTH reactions together by 1H- NMR and discuss their results for both compounds. · Each cyclohexanol is a mixture of TWO isomers: 50% cis and 50% trans. · There are TWO mechanisms to consider, E1 and E2. · You and your partner will collect TWO fractions from the distillation. Experiment 21 describes what the author calls the "Evelyn effect," which we will define as fraction 1 and 2 having a different r atio of products. Under the supervision of the TA, measure proton NMR spectra for each fraction and analyze the integration areas of the vinylic protons. As soon as you complete this NMR analysis for each fraction and compare the % compositions, you will easily see if they are different. The main part of your discussion is to explain the NMR r esults for each cyclohexanol mixture in terms of mechanism. For each of the two starting compounds, you must discuss the following six considerations in the order listed below. Use subheadings to separate your answers to each question clearly. State the percentage of 1-methylcyclohexene for each fraction. 1. Is there an Evelyn effect? 2. Is the Zaitsev rule followed? 3. Discuss possible rearrangements, and implications for the mechanism 4. Discuss reactions of the cis vs. trans compounds, the anti-periplanar requirement for E2, and implications for th e mechanism. 5. Explain the Evelyn effect, where it is observed. 6. Final conclusion: Is the mechanism E1, E2, or both? How would you use NMR integration to calculate relative percentages of components in a mixture? How do you choose which hydrogens to integrate? The integration areas of isolated hydrogens can be used to calculate relative percentages of components in a mixture. Calculating Percentages using Integration areas 0.95 x 100% = 50% 0.95+0.94 50:50 OR 1.10 1.10+0.90 x 100% 55:45 = 55% Average Value 52.5%:47.5% Simulated 1H NMR spectrum of 1methylcyclohexene Simulated 1H NMR spectrum of 3methylcyclohexene Simulated 1H NMR spectrum of 4methylcyclohexene Concepts to understand: 1) ester hydrolysis 2) ester formation from a carboxylic acid and alcohol – know the mechanisms! 3) interpretation of simple IR and mass spectra – be able to identify important fragment peaks in mass spectra or stretching or bending bands in IR spectra 4) chromatography principles a) gas chromatography b) column chromatography c) thin-layer chromatography