waterquality - Montana Pesticide Safety Education Program
advertisement

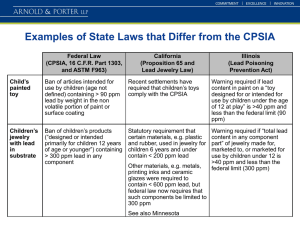
Cecil Tharp MSU Pesticide Education Program Bozeman, Montana That pesticide didn’t have any residual. I need to reapply every few weeks. That chemical doesn’t work at the labeled rates. I need to double or triple the rates! My pests are resistant to this chemical! They are full of it! That pesticide doesn’t work! Water Primary diluent New Term Why water quality is important! • 1 pint or 1 quart per acre • 30 GPA application volume • 99% and 93% of spray solution pH Hardness TDS Turbidity Alkalinity Is the measure of the concentration of hydrogen ions (H+) and hydroxide ions (OH-) in a solution. Scale is logarithmic from 0 - 14 Hydrogen predominates (acidic < 7) Hydroxide predominates (alkaline > 7) Dissociation is the breaking down of a complex molecule into simpler units. Efficacy often goes down when molecules are dissociated. Plants often won’t absorb the chemical as well. At times this may totally inactivate the pesticide. pH 6 – 7 is ideal for most pesticides but it can vary. Weak Acid Herbicides are the most susceptible to alkaline (high ph) dissociation Roundup® (glyphosate) Pursuit® (ammonium salt of imazethapyr) Liberty® (glufosinate ammonium). 2-4D salt At pH > 7, many organophosphate, pyrethroid, or carbamate insecticides can break-down in a matter of hours or minutes ‘Oxamyl’ Some pesticides are vulnerable to breaking down in a low pH solution. Sulfonyl urea (SU) herbicides (Escort, Ally) tend to break down more rapidly when the pH is < 6 (acid hydrolysis). This is more common in forested mountain areas of western MT. Consult the LABEL to see if there are any warnings about water pH. Know the pH of your water source. Purchase a “pocket pH meter” and use it or other testing kits which are widely available. Forestry Suppliers $55.95 Science Lab.Com $79.97 If > 8 or < 6 you may need to add buffering agent Use spray solution ASAP Add acidifier if needed Total Dissolve Solids (TDS) is the measure of all dissolved mineral cations and anions. Generally in Montana, hardness (cations only) is used to measure ionic water quality concerns related to pesticide performance. Gives water it’s taste Positive Charge (cations) Negative Charge (anions) Calcium (Ca++) Magnesium (Mg++) Sodium (Na+) Sulphate (SO4-) Chloride (Cl -) Bicarbonate (HCO3-) How is TDS measured? Calcium = 666 ppm Sulphate = 2434 ppm Magnesium = 234 ppm Chloride= 32 ppm Sodium = 130 ppm Bicarbonate = 346 ppm TOTAL = 3842 ppm. TDS > 500 mg/L (ppm) is salty to taste Types Of Water Drinking water <500 TDS (EPA) Fresh Water : <1,000 TDS Brackish : 1,000-5,000 TDS Highly Brackish : 5,000-15,000 TDS Saline : 15,000-30,000 TDS Sea Water : 30,000-40,000 TDS Brine : 40,000-300,000+ TDS Hardness is the concentration of multi-valent cations (positively charged ions from minerals) Cations bind with negatively charged pesticide molecules Positively charged pesticide molecules are converted to a negative charge when they are broken into smaller units from hydrolysis. pH and hardness work together to reduce efficacy Forms insoluble salts WHO Hardness Classification Chart If the sum of the concentration (ppm) for the cations exceeds 150 ppm action should be considered: 2,4-D amine (> 150 ppm) totally deactivated at 500 ppm) Dicamba (> 150 ppm) Glyphosate (> 150 ppm) Clopyralid (>150 ppm) sethoxydim ‘Poast’ (>150 ppm) Imazethapyr ‘Pursuit’ (>150 ppm) Reduces efficacy of many surfactants Scale may plug sprayer Add an adjuvant containing sulfate or organic acids as they bind with hard minerals non-ionic surfactants increase efficacy Add ammonium sulfate (8.5 – 17 lb / 100 gallons) Alkalinity or Bicarbonate Waters • • • • Associated with sodium > 500 ppm & high pH Affects 2,4-D Affects “dim” herbicides • Poast - sethoxydim • Select – clethodim • Achieve -tralkoxydim Bicarbonate Waters - Solution • Use maximum allowed rate • Apply during optimal growth stage • Adjuvants - Non-ionic Surfactant (NIS) - Acidifiers/buffers Turbid water, or water containing suspended solids, soil, or organic matter can reduce effectiveness of postemergence herbicides. Measured in Nephelometric Turbidity Units (mg/L) Many pesticides bind to soil particulates (high soil sorption potential) Glyphosate (24,000 KOC) Permethrin (100,000 KOC) Bifenthrin (240,000 KOC) Paraquat (1,000,000 KOC) Diquat Harbors microbes which can further break down pesticides Particulates can clog filters and nozzles Drop a quarter to the bottom of a 5 gallon bucket. If you can’t see the quarter then the water must be treated or not used. Locate an alternative water source Install inline filters Make sure intakes are not at bottom of tanks Check the water pH If greater than 7.5 consider buffering agents Especially if your pesticide is an organophosphate, carbamate, or a weak acid herbicide (product label pH warnings!) Test the hardness of your water If less than 6.0 (not likely) and using SU’s If over 150 ppm then consider adding adjuvants or alternative water source especially if using 2,4-D, glyphosate, dicamba, clopyralid Test the turbidity of your water If water is murky consider an alternative water source. Adjuvants • Additives to improve performance • Acidifiers • Buffers pH • Conditioners • Ammonium Sulfate (AS) • Non-ionic surfactants (NIS) Hard Water www.herbicide-adjuvants.com 1. Test the water 2. Reduce Water Volume 3. Use maximum allowed rate 4. Spray ASAP after mixing 5. Adjuvants Wilbur Ellis testing kits Great Falls Field Office (406-727-4500) Dillon Field Office (406-683-5355) Billings Field Office (406-248-1176) Navigate to Purdue University Water Quality webpage at http://www.ppp.purdue.edu/Pubs/PPP-86.pdf Gemplers.com Cecil Tharp Pesticide Education Specialist 406-994-5067 ctharp@montana.edu www.pesticides.montana.edu