Antacids
advertisement

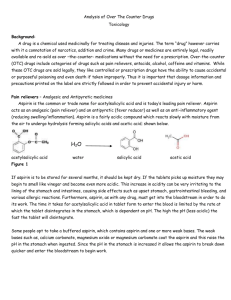
Antacids The walls of the human stomach contain cells which secrete gastric juices containing hydrochloric acid. The normal pH of gastric juices is in the 1.0 – 3.0 range. The purposes of this acidic solution are: to suppress growth of harmful bacteria, and to help in digestion by hydrolysing proteins to amino acids. Over-eating or eating certain types of food, or stress (worrying) stimulates excess acid production, causing indigestion. Antacid - a remedy for excess stomach acidity Antacids are bases - usually, metal oxides, hydroxides, carbonates or hydrogen carbonates (bicarbonates). they neutralize excess acid in the stomach to adjust the stomach pH to the desired level (they relieve indigestion and allow damage done by excess acid to the stomach lining to repair itself). Active ingredients in ‘over-thecounter’ antacids include: aluminium hydroxide Al(OH)3 Magnesium hydroxide Mg(OH)2 calcium carbonate CaCO3 Magnesium trisilicate MgSi3O8 and sodium hydrogen carbonate NaHCO3 Action of antacids 1. Magnesium oxide MgO (s) + 2 HCl (aq) MgCl2 (aq) + H2O (l) 2. Magnesium hydroxide Mg(OH)2 (aq) + 2 HCl (aq) MgCl2 (aq) +2 H2O (l) 3. Aluminium hydroxide Al(OH)3 (s) + 3 HCl (aq) AlCl3 (aq) + 3 H2O (l) 4. Calcium carbonate CaCO3 (s) + 2HCl (aq) CaCl2 (aq) + H2O (l) + CO2 (g) 5. Sodium hydrogen carbonate NaHCO3 (aq) + HCl (aq) NaCl (aq) + H2O (l) + CO2 (g) 6. Magnesium trisilicate Mg2Si3O8 (s) + 4 HCl (aq) 3 SiO2 (s) + 2 H2O (l) + 2 MgCl2 (aq) Antacids are often combined with chemicals called alginates that produce a neutralizing layer that prevents acid reflux. Similarly anti–foaming agents such as dimethicone are added that reduce the surface tension of gas bubbles, causing them to coalesce (come together), producing a defoaming action. Side effects of antacids Aluminum hydroxide may cause constipation or irregularity. Aluminum ions can also prevent uptake of phosphate ions, due to precipitation of aluminum phosphate. Also, they bind to certain drugs because of their large charge density due to their small ionic radius and high charge. Magnesium hydroxide has laxative properties. Calcium carbonate may result in kidney stones and sodium ions may lead to hypertension. Example Two solid antacid products containing the same mass of different active ingredients are on sale for the same price. One contains sodium bicarbonate, the other calcium carbonate as the active ingredient. Deduce which one is a better buy and explain your reasoning.