DAY 25: INTRO TO POLYMERS - Rose
advertisement

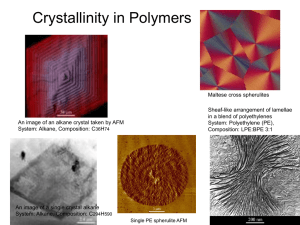
DAY 25: INTRO TO POLYMERS 1. 2. 3. 4. 5. 6. Our approach to this very important class of materials is as follows: Basic vocabulary and concepts Simple Polymers and their Properties Crystallinity in Polymers Mechanical Behavior of Polymers Polymer families Manufacturing issues We have about 2 weeks to work on this stuff. Please read text! WEB RESOURCE A really good website, comprehensive and more fun to read than the text is http://www.pslc.ws/macrog/index.htm CHAPTER 14 – POLYMERS What is a polymer? 3 Poly many mer repeat unit repeat unit repeat unit H H H H H H C C C C C C H H H H H H H H H H H H C C C C C C H Cl H Cl H Cl Polyethylene (PE) Polyvinyl chloride (PVC) repeat unit H C H H H C C CH3 H H H C C CH3 H H C CH3 Polypropylene (PP) Adapted from Fig. 14.2, Callister 7e. BASIC DEFINITIONS Polymers are huge molecules. They are sometimes found in nature, but nowadays are often produced by chemists and chemical engineers. Carbon, hydrogen, and other nonmetallic elements are important players. Covalent (primary) bonding exists within the molecule. Adjacent molecules are bonded with secondary bonds like Vanderwals and hydrogen bond. Can we make some predictions about density and strength based on the above? EFFECT OF BONDING ON PROPERTIES The primary bonds between the chains are strong mostly covalent bonds that you would expect to produce a Strong Stiff high melting temperature material. The secondary bonds mean that chains can move with respect to each other easily which makes polymers relatively Weak low stiffness low melting temperature DISCUSSION, POLYMERS (PE) VS. METALS AND CERAMICS Polymers are much less dense. This one floats, though not all do. Why? Polymers are much weaker. Why? Heavy dependence on the secondary bond. Polymers are much more ductile. Why? Lighter atoms, and not as efficiently packed. Chains can slide past one another. Again, secondary bond is temporary. Polymers are less stiff. Why? Way less crystallinity. MORE COMPARISONS The nature of the bonding – shared electrons – causes there to be no free electrons for conducting electricity. The mechanisms for conducting heat in polymers is also limited. Hence, these materials are insulators. THREE MAIN CATEGORIES Thermoplastics Primary bonds along the chains. Secondary bonding between chains. Elastomers These are chains that have some kind of strong (often primary bonds) between them. I.e. some “crosslinking”. Elastomers have some other features which will have to be discussed. Thermosets These are 3D network solids. Much primary bonding, little secondary. EXAMPLE OF A THERMOPLASTIC POLYMER: POLYETHYLENE Schematic of PE molecule C C Polyethylene mer Models of PE Ethylene CHEMISTRY OF POLYMERS Adapted from Fig. 14.1, Callister 7e. 10 Note: polyethylene is just a long HC - paraffin is short polyethylene EFFECT OF CHAIN LENGTH - PE Mer has 4 hydrogens and 2 carbons. MW = 28. LDPE (low density polyethylene) chain has MW of about 200,000 => 7000 mers. UHMWPE (ultra-high molecular weight PE) has MW between 3,000,000 and 6,000,000 => up to 200,000 mers. There are a large number of types of PE in between: 1. Medium Density PE (MDPE) 2. High Density PE (HDPE) 3. And many others. PE is a big family, and MW is part of that, but not the whole story. SOME PROPERTIES Material Density g/cc UTS Ksi %EL E ksi LDPE 0.917 2 600 18 MDPE 0.936 2.5 750 90 HDPE 0.953 4.5 200 240 UHMWPE 0.930 7 350 100 •COMPARE WITH METALS AND CERAMICS! •Note the UHMWPE does not have the highest density, but it does have the highest strength. •Note in general how the increase in density and molecular weight goes along with strength increases. MECHANICAL PROPERTIES OF POLYETHYLENE Type 1: (Branched) Low Density of 0.910 - 0.925 g/cc Type 2: Medium Density of 0.926 - 0.940 g/cc Type 3: High Density of 0.941 - 0.959 g/cc Type 4: (Linear) High Density to ultra high density > 0.959 13 Mechanical Properties Branched Low Density Density 0.91- 0.925 Medium Density 0.926- 0.94 High Density 0.941-0.95 Linear High Density 0.959-0.965 Crystallinity 30% to 50% 50% to 70% 70% to 80% 80% to 91% Molecular Weight Tensile Strength, psi Tensile Modulus, psi Tensile Elongation, % Impact Strength 10K to 30K 30K to 50K 50K to 250K 250K to 1.5M 600 - 2,300 1,200 - 3,000 3,100 - 5,500 5,000 – 6,000 25K – 41K 38K – 75 K 100% - 650% 100%- 965% 150K – 158 150K – 158 K K 10% - 1300% 10% - 1300% No break 1.0 – no break D50 – D60 ft-lb/in Hardness, Shore D44 – D50 0.4 – 4.0 0.4 – 4.0 D60 – D70 D66 – D73 www.csuchico.edu/~jpgreene/itec041/m41_ch06/m41_ch06.ppt WHY ARE LONGER CHAINS BETTER FOR STRENGTH AND STIFFNESS? Picture polymers as cooked spaghetti or boxes of wire or cable. If the spaghetti or cable is very short, it won’t entangle, but long lengths of cable stirred together will entangle severely. Entanglement means that strength and stiffness increase. EFFECT OF SIDE GROUPS PE had only hydrogen on the sides What happens when we put different elements or different groups of elements? POLAR SIDE GROUPS Polar side groups like Chlorine or Flourine can increase the strength of the secondary bonding. Bulky side groups like those on polypropylene increase the entanglement like barbed wire increases the entanglement of wire. Polymer Density g/cc UTS ksi %EL E ksi PVC 1.35 7.5 45 385 PP 0.950 5.0 150 300 PS 1.05 6.5 1.5 413 MDPE 0.936 2.5 750 90 EFFECT OF BACKBONE The all carbon backbone of PE is very flexible. The addition of N or O on the backbone can make it stiffer (harder to uncoil and slide past other chains (Nylon, Delrin) ) The existence of ring structures on the chain makes it really stiff. 22 Polymer Density UTS g/cc ksi %EL E ksi PVC 1.35 7.5 45 385 PP 0.950 5.0 150 300 PS 1.05 6.5 1.5 413 MDPE 0.936 2.5 750 90 Nylon 1.14 12 15-300 230-550 PC (Lexan) 1.2 10 120 345 ADVANTAGE OF CRYSTALLINITY Polymers have a limited ability to crystallize. Some, esp. PE are capable of forming crystalline structures. Over 90% crystalline. Some polymers have 0% crystallinity. They are totally amorphous. In PE, there is a crystal that forms by chain folding into sheets like this one. MORE ON CRYSTALLINITY The sheets form blade like structures which tend to grow outward from a common center into a spherical shape. This is called a spherulite. Please note that crystallinity is not 100%. PE CRYSTALS The spherulites grow together to give something like a polycrystalline grain. See the micrograph. EFFECTS OF CRYSTALLINITY Closer packing means stronger secondary bonds. Chain mobility and sliding is lessened. Add strength Adds stiffness, i.e Higher elastic modulus. Decreases ductility. WHAT FACTORS AFFECT CRYSTALLINITY? Branched chains don’t fold back and forth well. (linear PE is stronger than branched PE) Bulky Side Groups don’t fold back and forth well (polystyrene is amorphous) Location of side groups matters TWO TYPES OF PS SOME PROPERTIES OF A SYNDIOTACTIC PS Density: 1.11 g/cc UTS: 10.5 Ksi %EL 1.8% Modulus of Elasticity: 700 Ksi 1. 2. 3. Just a few remarks about the difference: Bulky side groups such as phenyl inhibit crystallinity. Sydndiotactic, ie regular placement on alt. sides promotes crystallinity. Crystallinity enhances strength and stiffness. BRANCHING Branching makes it harder for the polymers to lie next to each other and pack efficiently. Branched, looks like shorter chains! Strength will be lower and so will density in the branched IMPROVED PS – HIGH IMPACT PS (HIPS) We form what is called a “graft copolymer.” This is kind of like an alloy. Polybutadiene rubber chain Atactic polystyrene HIPS is strong and tough!!