L05C - Clarkson University
advertisement
L05C: Surface defects
• Defects arise in all stages of production and processing.
CASTING
• A common phase in production of metals is casting.
• A melt is put in a mold and heat is extracted through the mold wall.
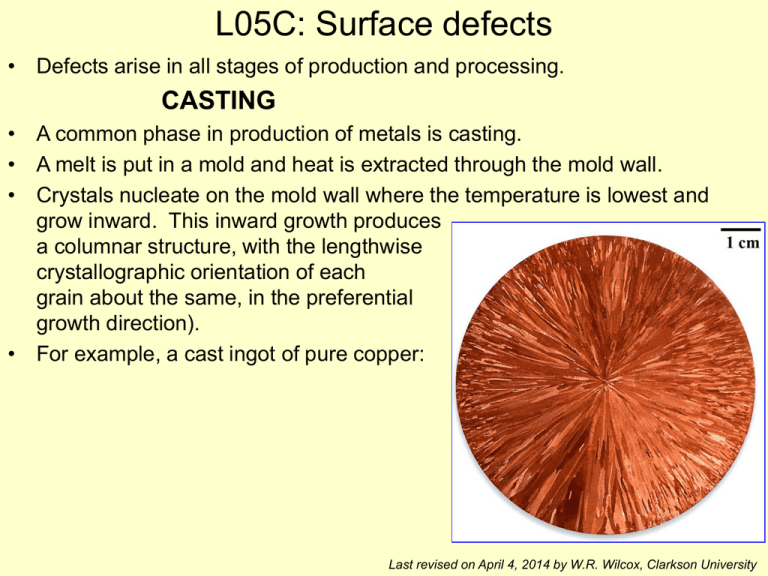
• Crystals nucleate on the mold wall where the temperature is lowest and
grow inward. This inward growth produces
a columnar structure, with the lengthwise
crystallographic orientation of each
grain about the same, in the preferential
growth direction).
• For example, a cast ingot of pure copper:
Last revised on April 4, 2014 by W.R. Wilcox, Clarkson University
Simulation of casting of an Al-Si alloy with
nucleation of new grains in the melt.
(http://www.tms.org/pubs/journals/jom/0201/thevoz/thevoz-0201.html )
View in projection mode to see the action.
Microstructure depends on many things:
• the alloy composition
• how close the melt is to the freezing point
when poured it
• how rapidly it's cooled
• whether the cooling's all around or mostly
on the bottom
•etc.
Grain Refiner - added to make smaller,
more uniform, equiaxed grains.
Casting of alloys
• Alloy crystals tend to grow as dendrites:
https://www.youtube.com/watch?v=S07fPo45BvM
• If the melt falls below its melting point while being added to the mold, small
crystals may have already nucleated in the melt and be floating around.
• Dendrite arms may detach and float around in the melt.
• After solidification is complete, grains formed by the floating crystals have
random shapes and orientation.
The region occupied by these in
the casting is called “equiaxed.”
• Example: Ti–47.2 Al–1.50:
http://www.sciencedirect.com/science/article/pii/S0966979507000817
Polycrystalline Materials
Grain Boundaries
• regions between crystals
• transition from lattice of one side
to that of the other
• High-angle grain boundaries
– highly disordered
– low density
– high impurity diffusivity
– high chemical reactivity
• Low-angle grain boundaries
– slightly disordered
– made up of a line of
dislocations, which can be
seen by usual methods of
revealing dislocations.
4
Low-angle grain boundaries
• If formed only by edge dislocations it’s a “tilt
boundary”
• If formed only by screw dislocations it’s a twist
boundary. Most are mixed.
• Dividing line between high-angle and lowangle boundaries is fuzzy, roughly between
10o and 20o
• If individual dislocations can be seen, can be
considered low angle.
• For example, etch pits on NaCl & YAlO3
Stacking faults
• Found in closed-packed face-centered cubic and hexagonal crystals
because only the second-nearest neighbors are different at the fault
• Reminder: Close-packed planes in FCC
are in order ABCABC {111}, while in HCP
the order is ABAB {0001}.
• Example: Austenitic steel:
• FCC–hexagonal stacking faults also
common with the diamond structure and
the zinc-blende structure. (Diamond &
Lonsdalite, zinc-blende & wurtzite.)
http://amadm.unileoben.ac.at/SFE_Steel.html
http://iopscience.iop.org/0953-8984/25/13/135002/article
Twin boundaries
http://en.wikipedia.org/wiki/Crystal_twinning
• Twins are two grains whose lattices are at a definite, reproducible,
orientation with respect to one another.
• Crystal lattices in the twins may be mirror images of one another, i.e.
reflection twins.
Si, for example: http://www.tf.uni-kiel.de/matwis/amat/def_en/kap_7/backbone/r7_1_1.html
• When the two lattices share all atoms at the boundary they are called
“coherent.” Common, but not always.
• Twinning can occur during plastic deformation, transformation to a different
crystal structure, or crystal growth.
• The mechanisms for twinning during deformation and transformation are
generally well understood.
• The mechanisms for twinning during crystal growth are generally unknown.
• Twin boundaries are often planar, and appear as straight lines in a section.
• But sometimes twin boundaries jog so that they appear curved at low
magnification.
• Twin boundaries are often parallel to one another.
Copper, for example: http://www.nature.com/am/journal/2009/200904/full/am2009128a.html
Examples of stacking faults & twins in metals
• The spheres labeled “A” in the figure to the
right from VMSE represent metal atoms in
a close-packed plane. Positions B and C
show the two possible locations for the
next close-packed plane on top of this one.
•
•
•
•
•
Planes stacked in the order ABCABC… generate a FCC crystal.
In FCC, one type of stacking fault can be represented by ABCBCABC.
In FCC, a reflection twin can be represented by ABCBCABC.
Planes stacked in the order ABABAB… generate a HCP crystal.
In HCP one type of basal plane (0001) stacking fault can be represented by
ABACABA
• Many twin planes observed in HCP and much more difficult to illustrate.
• More complex twins in BCC.
Interface between two phases
• Another type of surface defect. For example:
– Second phase inside the solid
– Thin films (extremely important technologically)
– Small solid particles in a gas or liquid.
• Notice that the interface may have a structure quite different from those of
the adjacent bulk phases (http://en.wikipedia.org/wiki/Surface_reconstruction)
• If we assume the crystal structure exists up to the surface, several types of
defects can exist at this surface:
Some chemical reactions may take
place only at specific surface sites.
Step
Solid Catalysts and Surface Defects
• A catalyst increases the
rate of a chemical reaction
without being consumed
• Active sites on catalysts
are normally surface
defects
Fig. 5.15, Callister & Rethwisch 4e.
Single crystals of
(Ce0.5Zr0.5)O2
used in an
automotive catalytic
converter
Fig. 5.16, Callister & Rethwisch 4e.
Volume defects
• Second phase in solid. Can be void, gas bubble, or another solid.
• When insoluble foreign particles are present in a melt, these may be trapped
in the solid during solidification.
• If the impurity is soluble in the solid at the melting point, it may precipitate
out as the solid is cooled. (Solid solubility normally decreases as
temperature is decreased.) These precipitates may be gas bubbles,
impurity itself, or compound between impurity and solid.
• Example: carbon flakes in
gray cast iron:
• Other methods of forming
composite materials:
• Mixing of concrete and then
hardening by formation of
hydrate crytals.
• Mixing of fibers with a
monomer and then
polymerizing.
http://www.metallographic.com/Technical/Metallography-Intro.html
Defects in Polymers
• Defects due in part to chain packing errors and impurities such
as chain ends and side chains
Adapted from Fig. 5.7,
Callister & Rethwisch 4e.
12
















