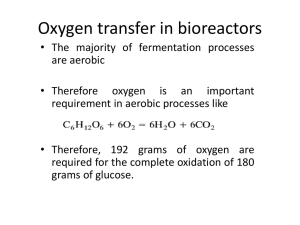
Temperature

Salinity and
Dissolved Oxygen (D.O.)
Lecture 2
Salinity defn
• Salinity is the saltiness or dissolved salt content of a body of water .
• fresh water (or sweet water) is less than 0.05%. brackish water is 0.03 to 0.05% salt by weight. ocean water is naturally saline at approximately
3.5% salt (see sea water ). over 5% it is considered brine .
• weight: 35 g/L (where 1L = 1.025kg approx)
• ppt: 35 ‰
• PSU: 35 (a dimensionless ratio)
Scale - units
• Prior to 1978, salinity was expressed as ‰ usually based on the electrical conductivity ratio of the sample to "Copenhagen water", an artificial sea water manufactured to serve as a world standard.
• In 1978, oceanographers redefined salinity in
Practical Salinity Units ( psu ): the conductivity ratio of a sea water sample to a standard KCl solution
Seawater
• On average, seawater in the world's oceans has a salinity of ~3.5%, or 35 parts per thousand.
This means that every 1 kg of seawater has approximately 35 grams of salts (mostly, but not entirely, sodium chloride ) dissolved in it. The average density of seawater at the surface of the ocean is 1.025 g / mL ; seawater is denser than fresh water (which reaches a maximum density of 1.000 g/mL at a temperature of 4 °C) because of the added weight of the salts
Estuarine Salinity
Measuring Salinity
• Refractometer
• Conductivity Meter
Refractometer
• http://en.wikipedia.org/wiki/Refractometer
• A refractometer is an optical instrument that is used to determine the refractive index of a substance or some physical property of a substance that is directly related to its refractive index. Certain types of refractometers can be used for measuring gases, liquids, and even transparent or translucent solids such as gemstones.
• A refractometer can be used to determine the identity of an unknown substance based on its refractive index, to assess the purity of a particular substance, or to determine the concentration of one substance dissolved in another. Most commonly, refractometers are used for measuring fluid concentrations such as the sugar content ( Brix level) of fruits, vegetables, juices and carbonated beverages http://www.atago.net/USA/mame.html
Conductivity
Salinity Based on Conductivity
The meters were very precise and relatively easy to use compared with the chemical techniques used to measure chlorinity. As a result, the Joint Panel also recommended that salinity be related to conductivity of sea water using:
S= -0.08996 + 28.2929729R
15
+ 12.80832 R 2
15
-10.67869R
3
15
+ 5.98624R
4
15
- 1.32311R
5
15
(6.3a)
R
15
= C(s,15,0)/C(35,15,0) (6.3b) where C (S, 15 , 0) is the conductivity of the sea-water sample at 15 °C and atmospheric pressure, having a salinity S derived from (6.4), and C (35 , 15 , 0) is the conductivity of standard "Copenhagen" sea water. Millero (1996) points out that (6.3) is not a new definition of salinity, it merely gives chlorinity as a function of conductivity of seawater relative to standard seawater. http://oceanworld.tamu.edu/resources/ocng_textbook/chapter06/chapter06_01.htm
All aqueous solutions conduct electricity to some degree. The measure of a solution’s ability to conduct electricity is called “conductance” and is the reciprocal of resistivity
(resistance). Adding electrolytes such as salts, acids or bases to pure water increases conductance (and decreases resistivity). A conductivity system measures conductance by means of electronics connected to a sensor immersed in a solution.
Historically, the unit of conductivity measurement has been the “mho/cm” (a mho is the multiplicative inverse of an ohm). A resistivity of 100 ohms x cm is equivalent to a conductivity of 1/100 mho/cm. The mho/cm unit of measurement is now being replaced in industry by an equal and interchangeable international unit called the “Siemen/cm.”
Conductivity is usually expressed in millionths of a Siemen, that is, in microSiemen/cm.
Resistivity is still expressed in terms of Megohm (MΩ) x cm for high purity water – usually from 0.1 to 20 MΩ x cm. (NOTE R.O. systems typically run 18 MΩ)
In measuring the 1 microSiemen/cm solution, the cell would be configured with large electrodes spaced a small distance apart. This results in a cell resistance of approximately
10,000 ohms, which can be measured quite accurately.
Because conductivity has a large temperature coefficient (as much as 4% per °C–see Fig. 1), an integral temperature sensor incorporated into its circuitry adjusts the reading to a standard temperature, usually 25 °C (77°F).
Conductivity measuring system accuracy is only as good as its temperature compensation. Since common solution temperature coefficients vary on the order of 1-3% per °C, measuring instruments with adjustable temperature compensation should be utilized.
CTD
CTD=Conductivity, Temperature, Depth the CTD is torpedo-shaped and may be deployed by itself, attached to a submersible, or as part of a larger metal water sampling array known as a rosette, or carousel. Multiple water sampling bottles are often attached to the rosette to collect water at different depths of the cast. Sometimes, the metal frame is attached to a conducting wire and lowered into the water. Information is sent back to the ship along the wire while the instrument is lowered (downcast) to a depth specified by the scientist and then brought back (upcast) to the surface. In other cases, the CTD can store the data collected in its memory, which can be downloaded and analyzed at a later time using software similar to that developed for sondes.
Dissolved Oxygen
• Required to sustain life.
• TEMPERATURE DEPENDANT!!!!
• Colder water can hold more oxygen.
• Range is 0 – 12 mg/L (=ppm)
• Hypoxia (less than 2 mg/L)
• Anoxia (less than 0.5 mg/L)
• Bacteria are major users of D.O.
• Photosynthesis is a major source of D.O. (but only during the day! – needs to have light).
At 20º C (room temperature) and standard atmospheric pressure (sea level), the maximum amount of oxygen that can dissolve in fresh water is 9 ppm. If the water temperature is below 20º C, there may be more oxygen dissolved in the sample. In general, a dissolved oxygen level of 9-10 ppm is considered very good.
• Why Dissolved Oxygen is Important
• Dissolved oxygen analysis measures the amount of gaseous oxygen (O2) dissolved in an aqueous solution. Oxygen gets into water by diffusion from the surrounding air, by aeration (rapid movement), and as a waste product of photosynthesis. Oxygen dissolves in water at very low concentrations.
Our atmosphere is 20% oxygen or 200,000 ppm but seldom will a pond have more than 10 ppm oxygen dissolved in its' water.
• When performing the dissolved oxygen test, only grab samples should be used, and the analysis should be performed immediately. Therefore, this is a field test that should be performed on site.
•
Environmental Impact:
• Total dissolved gas concentrations in water should not exceed 110 percent.
Concentrations above this level can be harmful to aquatic life. Fish in waters containing excessive dissolved gases may suffer from "gas bubble disease"; however, this is a very rare occurrence. The bubbles or emboli block the flow of blood through blood vessels causing death. External bubbles
(emphysema) can also occur and be seen on fins, on skin and on other tissue. Aquatic invertebrates are also affected by gas bubble disease but at levels higher than those lethal to fish.
• Adequate dissolved oxygen is necessary for good water quality. Oxygen is a necessary element to all forms of life. Natural stream purification processes require adequate oxygen levels in order to provide for aerobic life forms. As dissolved oxygen levels in water drop below 5.0 mg/l, aquatic life is put under stress. The lower the concentration, the greater the stress. Oxygen levels that remain below 1-2 mg/l for a few hours can result in large fish kills.
http://aquaplant.tamu.edu/contents/dissolved_oxygen.htm
http://www.state.ky.us/nrepc/water/wcpdo.htm
D.O. (% saturation vs mg/L)
• Dissolved Oxygen Percent Saturation
• Percent Saturation is the amount of oxygen dissolved in the water sample compared to the maximum amount that could be present at the same temperature. For example, water is said to be 100% saturated if it contains the maximum amount of oxygen at that temperature. A water sample that is
50% saturated only has half the amount of oxygen that it could potentially hold at that temperature. Sometimes water can become supersaturated with oxygen because of rapidly tumbling water. This usually lasts for a short period of time but can be harmful to fish and other aquatic organisms. DO
Percent Saturation values of 80-120% are considered to be excellent and values less than 60% or over 125% are considered to be poor.
• The Percent Saturation of Dissolved Oxygen depends on the temperature/salinity of the water and elevation of the water testing site. Determine the altitude (elevation) or atmospheric pressure and use the table at
( http://www.ciese.org/curriculum/dipproj2/en/fieldbook/saturation.shtml
) to determine the correction factor. Multiply the Dissolved Oxygen level (in ppm) by the correction factor.
Measuring Dissolved Oxygen
• Winkler titration – very exact
• Clark polarographic membrane - fast
• Luminescent probe – the new wave
Winkler titration method
• Wet chemical method. Uses nasty chemicals. Very precise if done right.
• Sample + Manganous sulfate + Potassium hydroxide +
Potassium Iodide: O2 binds to Mn to create Mn (III), however this is UNSTABLE, so it is fixed with Sulfuric
Acid.
• Manganic Sulfate acts as strong oxidizer to release
Iodine.
• This “free” iodine is tritrated with Sodium Thiosulfate and
Starch until the soln turns from blue to clear. http://en.wikipedia.org/wiki/Winkler_test_for_dissolved_oxygen
Clark membrane
• Amperometric measurement = needs electrical power.
• Power flows from Anode (silver) to Cathode
(gold) via an electrolyte soln (KCl).
• A thin semi-permeable membrane allows O2 to enter cell.
• O2 reduced on cathode:
• Rate of reduction = rate of O2 diffusion, which is proportional to O2 concentration.
• Take about 2 mins to warm up and stabilize
(YSI)
LDO meter = optode
• Blue excitation light used to excite the luminophor
(fluorescent compound).
• Measure the red “fluorescent” light.
• The rate of at which blue light excitation is converted to red light fluorescence is inversely proportional to the D.O. concentration.
• Fast response = high D.O.
Lab
• Learn to prepare and check a Clark membrane probe for proper operations.
• Compare to an LDO probe.
• Use a refractometer and conductivity meter.
• Learn how to calibrate D.O. probes.