Chemistry Calculations - franklychemistry.co.uk.
advertisement

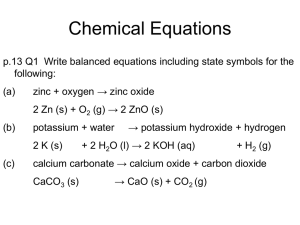
Remember that you can search using “edit”! GCSE Questions and Answers Calculations 6 consecutive GCSE Chemistry papers: 2002-7 1 I 1 H 1 Relative Atomic Masses you’ll need. II Atomic Number at the top Relative Atomic Mass at the bottom Ammonium 3 4 Li Be 7 9 11 12 Na Mg 23 24 III IV V VI VII VIII 2 He 4 NH4+ Hydroxide Nitrate Hydrogencarbonate Hydrogen sulphate OHNO3HCO3HSO4- Carbonate Sulphate CO32SO42- 5 6 7 8 9 10 B C N O F Ne 11 12 14 16 19 20 13 14 15 16 17 18 Al Si P S Cl Ar 27 28 21 32 35.5 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 2 Covalent elements and Compounds Covalent Elements: Covalent Compounds Hydrogen Chlorine Bromine Iodine Oxygen Nitrogen Helium Neon Argon H2 Cl2 Br2 I2 O2 N2 He Ne Ar Water H2O Carbon dioxide CO2 Carbon monoxide CO Sulphur dioxide SO2 Sulphur trioxide SO3 Ammonia NH3 Hydrogen peroxide H2O2 Nitrogen monoxide NO Nitrogen dioxide NO2 Sulphur Phosphorus S8 P4 3 2002, Paper 1 To obtain full marks in this question, you must show your working out 5 When washing soda crystals, Na2CO3.10H2O, are left exposed to the atmosphere they lose water of crystallisation. The longer they are left, the more water is lost. The amount of water of crystallisation remaining can be found in two ways: either by heating to remove all the remaining water or by titration. 5a) 2.675g of a sample of crystals were heated to constant mass. The mass of the residue was 1.325g. (i) Why was the sample heated to constant mass? ______________________________ [1] (ii) Calculate the number of moles of anhydrous sodium carbonate in the residue. ______________________________ [2] Consequential marking applies throughout 5a) (i) (ii) 2.675g of a sample of crystals were heated to constant mass. The mass of the residue was 1.325g. Why was the sample heated to constant mass? To ensure that all water [1] (of crystallisation) was lost. Calculate the number of moles of anhydrous sodium carbonate in the residue. Na2CO3 = 1.325 = 0.0125 [1] 106 [1] (iii) Calculate the mass of water lost and from this calculate the number of moles of water lost. _________________________________ ______________________________ [3] (iv) From your answers to part (a)(ii) and (iii) above, calculate the value of x in the formula Na2CO3.xH2O. _________________________________ ______________________________ [2] (iii) Calculate the mass of water lost and from this calculate the number of moles of water lost. Mass of water lost = (2.765-1.325) g = 1.35g [1] Number of moles lost = 1.35 = 0.075 [1] 18 [1] (iv) From your answers to part (a)(ii) and (iii) above, calculate the value of x in the formula Na2CO3.xH2O. Ratio of moles Na2CO3 : H2O 0.0125 : 0.075 [1] 1 : 6 [1] b) 1.775g of a different sample of washing soda was dissolved in distilled water and made up of a total volume of 250cm3. 25.0cm3 of this solution were titrated with 0.08 mol/dm3 (moles per litre) nitric acid. 31.25cm3 of acid were required. The equation for the reaction is: Na2CO3 + 2HNO3 → 2NaNO3 + H2O + CO2 (i) Calculate the number of moles of nitric acid used in the titration. ______________________________________ ___________________________________ [2] b) 1.775g of a different sample of washing soda was dissolved in distilled water and made up of a total volume of 250cm3. 25.0cm3 of this solution were titrated with 0.08 mol/dm3 (moles per litre) nitric acid. 31.25cm3 of acid were required. The equation for the reaction is: Na2CO3 + 2HNO3 → 2NaNO3 + H2O + CO2 (i) Calculate the number of moles of nitric acid used in the titration. Number of moles HNO3 = 31.25 x 0.08 1000[1] = 2.5 x 10-3 (ii) Calculate the number of moles of sodium carbonate present in the 25.0cm3 sample. _______________________________________ ____________________________________[2] (iii) Calculate the number of moles of sodium carbonate present in 250cm3 of solution. _______________________________________ ____________________________________[2] (ii) Calculate the number of moles of sodium carbonate present in the 25.0cm3 sample. Na2CO3 + 2HNO3 → 2NaNO3 + H2O + CO2 mole ratio 1 : 2 [1] Number of moles Na2CO3 = 2.5 x 10-3 2 = 1.25 x 10-3 (iii) Calculate the number of moles of sodium carbonate present in 250cm3 of solution. Number of moles Na2CO3 in 250cm3 = 1.25 x 10-3 x 10 [1] = 1.25 x 10-2 [1] (iv) Using your answer to part (b)(iii) and the mass of sodium carbonate crystals weighed out find the value of x in the formula Na2CO3.xH2O. _________________________________ ______________________________[2] (iv) Using your answer to part (b)(iii) and the mass of sodium carbonate crystals weighed out find the value of x in the formula Na2CO3.xH2O. Number of moles = moles rfm rfm = mass = 1.775 no. of moles 1.25 x 10-2 = 142 [1] rfm Na2CO3 = 106 rfm x H2O = 142 – 106 =36 [1] x = 36 = 2 [1] 18 c) Sodium hydrogencarbonate decomposes when it is heated into sodium carbonate according to the equation: 2NaHCO3 → NaCO3 + H2O + CO2 1.68g of sodium hydrogencarbonate were placed in a test tube and heated in a Bunsen flame for some time. (i) Calculate the number of moles of sodium hydrogencarbonate used. _______________________________________ ____________________________________ [2] c) Sodium hydrogencarbonate decomposes when it is heated into sodium carbonate according to the equation: 2NaHCO3 → NaCO3 + H2O + CO2 1.68g of sodium hydrogencarbonate were placed in a test tube and heated in a Bunsen flame for some time. (i) Calculate the number of moles of sodium hydrogencarbonate used. Number of moles NaHCO3 = 1.68 84 [1] = 0.02 [1] (ii) Calculate the number of moles of sodium carbonate formed. ___________________________________ ________________________________ [2] (iii) Calculate the mass of sodium carbonate expected to be formed. ___________________________________ ________________________________ [2] (ii) Calculate the number of moles of sodium carbonate formed. Mole ratio NaHCO3 : Na2CO3 2 : 1 [1] number of moles Na2CO3 formed = 0.02 = 0.01 [1] 2 (iii) Calculate the mass of sodium carbonate expected to be formed. Mass Na2CO3 expected = 0.01 x 106 [1] = 1.06g [1] (iv) Calculate the volume of carbon dioxide produced in this experiment. (1 mole of gas occupies 24dm3 at room temperature and pressure) _____________________________________ __________________________________ [2] (iv) Calculate the volume of carbon dioxide produced in this experiment. (1 mole of gas occupies 24dm3 at room temperature and pressure) Mole ratio NaHCO3 : CO2 2 : 1 [1] Number of moles CO2 expected = 0.02 = 0.01 [1] Volume of CO2 expected = 24 x 0.01 = 0.24dm3 [1] or 240cm3 2003, Paper 1 4 To obtain full marks in this question, all steps in the calculation must be shown. a) Copper carbonate, CuCO3 decomposes on heating according to the following equation: CuCO3 → CuO + CO2 (Relative atomic masses: C = 12; O = 16; Cu = 64) The following results were obtained in an experiment in which a sample of copper carbonate was to be heated. Mass of empty crucible = 24.21g Mass of crucible and sample of copper carbonate = 27.31g (i) What colour change would you observe on heating the sample of copper carbonate? _______________________________ [2] (ii) Calculate the mass of copper carbonate in the crucible. _______________________________ [1] (i) What colour change would you observe on heating the sample of copper carbonate? Green [1] to black [1] [2] (ii) Calculate the mass of copper carbonate in the crucible. 27.31 – 24.21 = 3.1g [1] (iii) Calculate the mass of solid copper oxide, CuO, which you would expect to obtain from the complete decomposition of this sample of copper carbonate. (iii) Calculate the mass of solid copper oxide, CuO, which you would expect to obtain from the complete decomposition of this sample of copper carbonate. 3.1 = 0.025 [1] moles of CuCO3 124[1] CuCO3 : CuO 1:1 [1] 0.025 [1] moles of CuO 0.025 x 80 [1] = 2.0 [1]g (iv) How would you ensure all the copper carbonate had decomposed? _______________________________ [1] (iv) How would you ensure all the copper carbonate had decomposed? Heat to constant mass [1] b) The experiment was carried out, heating the sample for 3 minutes. Not all of the copper carbonate had decomposed in this time. The results obtained are shown below: Mass of empty crucible = 24.21g Mass of crucible and sample of copper carbonate = 27.31g Mass of crucible and solid after heating for 3 minutes = 26.43g (i) What mass of solid remained after heating for 3 minutes? _______________________________ [2] (ii) Calculate the mass of carbon dioxide gas lost in this experiment. _______________________________ [2] (i) What mass of solid remained after heating for 3 minutes? 26.43 – 24.21 [1] = 2.22 [1]g (ii) Calculate the mass of carbon dioxide gas lost in this experiment. 27.31 – 26.43 [1] = 0.88 [1] g (iii) Calculate the number of moles of carbon dioxide gas lost in this experiment. _______________________________ [2] (iv) Calculate the mass of copper carbonate which must have been decomposed in this experiment. __________________________________ _______________________________ [ ] (iii) Calculate the number of moles of carbon dioxide gas lost in this experiment. 0.88 44[1] = 0.02 [1] moles of CO2 (iv) Calculate the mass of copper carbonate which must have been decomposed in this experiment. CO2 : CuCO3 = 1 : 1 0.02 [1] moles of CuCO3 decomposed 0.02 x 124 = 2.48 [1]g of CuCO3 decomposed v) Using your answer to parts (a)(ii) and (b)(iv), calculate the percentage of copper carbonate in the original sample which must have been decomposed in this experiment. [2] v) Using your answer to parts (a)(ii) and (b)(iv), calculate the percentage of copper carbonate in the original sample which must have been decomposed in this experiment. 2.48 3. 1 x 100 [1] = 80% [2] c) Copper carbonate reacts with sulphuric acid and crystals of hydrated copper sulphate, CuSO4.5H2O, can be obtained. Calculate the percentage of water of crystallisation in hydrated copper sulphate, CuSO4.5H2O. (Relative atomic masses: H=1; O=16; Cu=64) [3] c) Copper carbonate reacts with sulphuric acid and crystals of hydrated copper sulphate, CuSO4.5H2O, can be obtained. Calculate the percentage of water of crystallisation in hydrated copper sulphate, CuSO4.5H2O. (Relative atomic masses: H=1; O=16; Cu=64) [3] CuSO4.5H2O RFM = 250 [1] Mass of water = 18 x 5 = 90 [1] Percentage water = 90 x 100 =36% [1] 250 d) Carbon dioxide reacts with excess sodium hydroxide solution according to the following equation: CO2 + 2NaOH → Na2CO3 + H2O (1 mole of any gas at room temperature and pressure occupies 24 000cm3) 120cm3 of carbon dioxide gas is passed into 150cm3 of 0.1mol/dm3 moles per litre.mol/l sodium hydroxide solution. (i) Calculate the number of moles in 120cm3 of carbon dioxide gas. _____________________________________ __________________________________ [2] (i) Calculate the number of moles in 120cm3 of carbon dioxide gas. 120 24000 [1] = 0.005 [1] moles CO2 (ii) Calculate the number of moles of sodium hydroxide needed to react with this amount of carbon dioxide. _____________________________________ __________________________________ [2] (ii) Calculate the number of moles of sodium hydroxide needed to react with this amount of carbon dioxide. CO2 : NaOH [1] 0.005 moles x 2 = 0.01 moles NaOH needed [1] (iii) Calculate the number of moles in 150cm3 of 0.1mol/dm3 sodium hydroxide solution. _____________________________________ __________________________________ [2] (iii) Calculate the number of moles in 150cm3 of 0.1mol/dm3 sodium hydroxide solution. 150x0.1 1000 [1] = 0.015 [1] moles NaOH [2] (iv) How many moles of sodium hydroxide are left at the end of the reaction? _____________________________________ __________________________________ [2] (iv) How many moles of sodium hydroxide are left at the end of the reaction? Moles NaOH needed to react with CO2 = 2 x 0.005 = 0.01 Moles NaOH remaining = 0.015 – 0.01 [1] = 0.005 [1] e) Sodium hydroxide can be neutralised by hydrochloric acid according to the equation. NaOH + HCl → NaCl + H2O Using your answer to part (d)(iv) calculate the number of 0.5 mol/dm3 hydrochloric acid which would be required to neutralise the sodium hydroxide left at the end of the reaction. _____________________________________ __________________________________ [4] e) Sodium hydroxide can be neutralised by hydrochloric acid according to the equation. NaOH + HCl → NaCl + H2O Using your answer to part (d)(iv) calculate the number of 0.5 mol/dm3 hydrochloric acid which would be required to neutralise the sodium hydroxide left at the end of the reaction. NaOH : HCl = 1.1 [1] moles HCl required = 0.005 [1] volume HCl required = 0.005x1000 0.5 [1] = 10 [1] cm3 2004, Paper 1 5 To obtain full marks in this question, all steps in the calculation must be shown. a) In 1908 a German chemist called Fritz Haber succeeded in combining nitrogen with hydrogen to form ammonia. N2 + 3H2 → 2NH3 Calculate the volume of nitrogen gas, measured at room temperature and pressure, needed to produce 10dm3 of ammonia. Calculate the volume of nitrogen gas, measured at room temperature and pressure, needed to produce 10dm3 of ammonia. 1 volume of nitrogen needs 2 volumes of ammonia [1] hence 10dm3 needs 5dm3/4.99 [1] dm3 b) A concentrated solution of ammonia can be used as a fertiliser. To determine the concentration of the ammonia it was first diluted by measuring 10.0cm3 and making the volume up to 1dm3 (1000cm3). A 25.0cm3 sample of this dilute ammonia solution was then titrated against 0.05 mol/dm3 (moles per litre) sulphuric acid. The 25.0 cm3 of diluted ammonia required 12.5cm3 of the acid for neutralisation. The equation for the titration is 2NH3 + H2SO4 → (NH4)2SO4 (i) Calculate the number of moles of sulphuric acid used in the titration. (ii) Calculate the number of moles of ammonia in the 25.0cm3 sample which reacted with the acid. (i) Calculate the number of moles of sulphuric acid used in the titration. Number of moles of sulphuric acid = 12.5 x0.05 1000 = 0.000625 [2] (ii) Calculate the number of moles of ammonia in the 25.0cm3 sample which reacted with the acid. From equation (1:2) 1 mole sulphuric acid reacts with 2 moles of ammonia [1] 0.000625 moles acid reacts with 0.00125 moles ammonia [1] (iii) Calculate the concentration of the dilute ammonia solution in mol/dm3 (moles per litre). (iv) Calculate the concentration of the original concentrated ammonia solution in mol/dm3 (moles per litre). (iii) Calculate the concentration of the dilute ammonia solution in mol/dm3 (moles per litre). Concentration of ammonia = 0.00125x1000 25 = 0.05 [1] mol/dm3 (iv) Calculate the concentration of the original concentrated ammonia solution in mol/dm3 (moles per litre). Diluted 100 times [1] original conc = 0.05 x 100 = 5 [1] mol/dm3 (v) Calculate the concentration of the original concentrated ammonia solution in g/dm3 (v) Calculate the concentration of the original concentrated ammonia solution in g/dm3 RFM of NH3 = 17[1] conc g/dm3 = 5 x 17[1] = 85[1] g/dm3 c) Solid fertilisers are easier to store, hence fertilisers like solid ammonium chloride are preferred over ammonia solution. To produce ammonium chloride, ammonia is reacted with hydrochloric acid, according to the equation below. NH3 + HCl → NH4Cl What mass of ammonium chloride is formed when 73g of hydrochloric acid are completely neutralised by ammonia? (Relative atomic masses: H=1, N=14, Cl=35.5 What mass of ammonium chloride is formed when 73g of hydrochloric acid are completely neutralised by ammonia? (Relative atomic masses: H=1, N=14, Cl=35.5 RFM of HCl = 36.5 [1] moles of HCl = 73/36.5 = 2[1] (ratio 1:1 hence) moles NH4Cl = 2[1] RFM of NH4Cl = 53.5[1] 2 x 53.5 = 107g[1] d) Another important fertiliser made from ammonia is urea. It contains 20.00% carbon, 6.66% hydrogen, 46.67% nitrogen and 26.67% oxygen. Calculate the formula of urea. (Relative atomic masses: H=1, C=12, N=14, O=16) d) Another important fertiliser made from ammonia is urea. It contains 20.00% carbon, 6.66% hydrogen, 46.67% nitrogen and 26.67% oxygen. Calculate the formula of urea. (Relative atomic masses: H=1, C=12, N=14, O=16) In 100g of compound there are: 20/12 moles C = 1.67 [1] 6.67/1 moles H = 6.67 [1] 46.67/14 moles N = 3.33[1] 26.67/16 moles O = 1.67[1] formula is CH4N2O [1] accept any correct whole number multiple. e) Car exhaust fumes contain harmful nitrogen monoxide gas. Research has shown that when a stream of ammonia gas is injected into the hot exhaust a reaction occurs which converts the harmful nitrogen monoxide, NO, to nitrogen gas according to the equation below. 6NO + 4NH3 → 5N2 + 6H2O (i) How many moles of ammonia would be needed to react with 0.6 moles of nitrogen monoxide, NO? ___________________________ [1] (i) How many moles of ammonia would be needed to react with 0.6 moles of nitrogen monoxide, NO? 0.4 moles [1] (ii) The average car emits 0.033 moles of nitrogen monoxide per km. How many moles of ammonia would be needed to convert this to N2 gas? ___________________________ [2] (ii) The average car emits 0.033 moles of nitrogen monoxide per km. How many moles of ammonia would be needed to convert this to N2 gas? 6 moles NO: 4 moles NH3/0.033moles NO: 0.033/6x4 [1] = 0.022 [1] moles per km (iii) Using your answer to (e)(ii) calculate the mass of ammonia needed to convert 0.033 moles of NO to N2 gas. ___________________________ [2] (iii) Using your answer to (e)(ii) calculate the mass of ammonia needed to convert 0.033 moles of NO to N2 gas. 0.022 x 17[1] = 0.374g [1] 2005, Paper 2 2 Lead is extracted from the ore galena, PbS. a) The ore is roasted in air to produce lead(II) oxide, PbO. 2PbS(s) + 3O2(g) → 2PbO(s) + 2SO2(g) (Relative Atomic Masses: Pb=207, S=32, O=16) (i) Calculate the mass of lead(II) oxide, PbO, produced from 2390g of galena, PbS. (Show all steps in your calculations. [5] (i) Calculate the mass of lead(II) oxide, PbO, produced from 2390g of galena, PbS. (Show all steps in your calculations. [5] RFM PbS = 207+32 = 239 [1] 2390 Moles PbS = 239 = 10 [1] Moles PbS = 10 [1] RFM PbO = 270+16 = 223 [1] Mass PbO = 10x223 = 2230g or 2.23kg [1] The lead(II) oxide is reduced to lead by heating it with carbon in a blast furnace. PbO(s) + C(s) → Pb(l) + CO(g) The molten lead is tapped off from the bottom of the furnace. (ii) Using your answer to part (a)(i), calculate the mass of lead that would eventually be produced. (ii) Using your answer to part (a)(i), calculate the mass of lead that would eventually be produced. PbO: Pb = 1:1 [1] Moles Pb = 10 [1] Mass Pb = 10 x 270 = 2070g or 2.07kg [1] b) Lead metal forms several oxides. The formula of lead oxide may be represented as PbxOy. In an experiment to find the formula of a sample of lead oxide, a porcelain dish was weighed and the mass recorded. The porcelain dish was then filled with the lead oxide and reweighed. The mass was again recorded. The dish was placed in a hard-glass tube and heated in a stream of hydrogen gas. The hydrogen reduced all of the lead oxide to a bead of silvery lead metal. The apparatus was allowed to cool and the dish and its contents were reweighed. (i) Calculate the mass of lead metal produced. _____________________________________ [1] (ii) Calculate the mass of oxygen present in the lead oxide. _____________________________________ [1] (i) (ii) Calculate the mass of lead metal produced. 27.56 – 21.35 = 6.21g [1] Calculate the mass of oxygen present in the lead oxide. 28.20 – 27.56 = 0.64g [1] (iii) Using your answers to (i) and (ii), calculate the formula of the sample of lead oxide. (Relative atomic masses: Pb=207, O=16) (iii) Using your answers to (i) and (ii), calculate the formula of the sample of lead oxide. (Relative atomic masses: Pb=207, O=16) Moles Pb = = 0.03 [1] Moles O = = 0.04 [1] Ratio 3:4 so formula is Pb3O4 [1] c) Titration is a technique used by chemists to find the concentration of a solution. The apparatus used in a titration is shown opposite. (i) Identify the pieces of apparatus A and B. A is a ________________________________ [1] B is a ________________________________ [1] (ii) Describe in detail, stating precautions to ensure safety and accuracy, how you would transfer 25.0cm3 of an alkali into the conical flask using the piece of apparatus A. _____________________________________ _____________________________________ _____________________________________ _____________________________________ [3] (i) (ii) Identify the pieces of apparatus A and B. A is a pipette B is a burette [1] [1] Describe in detail, stating precautions to ensure safety and accuracy, how you would transfer 25.0cm3 of an alkali into the conical flask using the piece of apparatus A. Rinse with deionised water [1] rinse with alkali [1] use safety pipette filler/safety goggles [1] to draw up liquid until bottom of meniscus on line [1] release [1] into conical flask touch tip of pipette to surface of alkai [1] (Max [3]) (iii) Describe in detail, stating precautions to ensure accuracy, the steps you would take to prepare the piece of apparatus B for use in a titration. _______________________________ _______________________________ _______________________________ _______________________________ [4] (iii) Describe in detail, stating precautions to ensure accuracy, the steps you would take to prepare the piece of apparatus B for use in a titration. Rinse with deionised water [1] rinse with solution [1] fill burette with solution [1] use funnel [1] ensure jet is filled [1] ensure no air bubbles [1] (Max [4]) d) Limewater is calcium hydroxide solution. In a titration to find the concentration of calcium hydroxide in limewater, 25.0cm3 of limewater required 16.4cm3 of hydrochloric acid of concentration 0.040 mol/dm3 for neutralisation. (Relative atomic masses: Ca=40, O=16, H=1) Ca(OH)2 + 2HCl → CaCl2 + 2H2O (i) Calculate the concentration of the calcium hydroxide in mol/dm3 (mol per litre). Answer __________________ mol/dm3 [4] (ii) Calculate the concentration of the calcium hydroxide in g/dm3 (grams per litre). Answer __________________ mol/dm3 [4] (i) Calculate the concentration of the calcium hydroxide in mol/dm3 (mol per litre). Answer 16.4 x0.04 1000 = 0.000656 [1] Moles Ca(OH)2 = 0.000328 25 (ii) 0.000656 2 0.000328 [1] x 1000 [1] = 0.01312 mol/dm3 Calculate the concentration of the calcium hydroxide in g/dm3 (grams per litre). Answer Mass = mol x RFM = 0.01312 x 74 [1] = 0.971 [1] g/dm3 2006, Paper 2 7a) Oxygen forms ozone gas, O3, in the upper atmosphere according to the equation: 3O2(g) → 2O3(g) 150m3 of oxygen reacts completely to form ozone. (i) State Avogadro’s Law _______________________________ _______________________________ _______________________________ [3] (i) State Avogadro’s Law Equal volumes of gas [1] under the same conditions of temperature and pressure [1] contain the same number of particles [1] or moles of ozone = 4.2 moles [3] (ii) Using Avogadro’s Law or otherwise, calculate the volume of ozone gas produced. ____________________________m3 [2] (ii) Using Avogadro’s Law or otherwise, calculate the volume of ozone gas produced. 150 x2[1] 100[1]m3 3 [2] b) In the laboratory ozone gas can be produced by passing an electrical discharge through dry air. 450cm3 of ozone gas is produced when the temperature is 300K and the pressure is 1 atmosphere. The ozone gas is compressed using a pressure of 8 atmospheres and the temperature is decreased to 200K. Calculate the volume of ozone gas under these new conditions. _______________________________cm3 [4] b) Calculate the volume of ozone gas under these new conditions. P1V1 P2V2 [1] T1 T2 8 xV 1x450 2 [1] 300 200 1x450 x200 V2 [1] 300 x8 3 V 2 37 . 5 [ 1 ] cm _______________________________ [4] c) Ozone is used in very small amounts in underground railway stations to remove compounds which cause stations to be stuffy. One of the compounds which is formed is formaldehyde CH2O. Calculate the percentage by mass of carbon in CH2O. ____________________________% [3] c) Calculate the percentage by mass of carbon in CH2O. RFM (CH2O) = 30 [1] % carbon = 12 x100 30 [1] = 40% [1] [3] d) 1.92g of sulphur dioxide SO2, reacts completely with ozone to form 2.40g of sulphur trioxide, SO3. (i) Calculate the number of moles of sulphur dioxide used. _______________________________ [2] d) 1.92g of sulphur dioxide SO2, reacts completely with ozone to form 2.40g of sulphur trioxide, SO3. (i) Calculate the number of moles of sulphur dioxide used. RFM (SO2) = 64 [1] Moles = 1.92 64 = 0.03 [1] [2] (ii) Calculate the mass of ozone which reacts. _______________________________ [1] (iii) Calculate the number of moles of ozone which reacts. _______________________________ [2] (ii) Calculate the mass of ozone which reacts. 2.4 – 1.92 = 0.48g [1] (iii) Calculate the number of moles of ozone which reacts. RFM (O3) = 48[1] Moles = 0.48 48 = 0.01 [1] [2] (iv) Calculate the number of moles of sulphur trioxide formed. _______________________________ [2] (iv) Calculate the number of moles of sulphur trioxide formed. RFM (SO3) = 80[1] 2.4 Moles = 80 = 0.03[1] [2] (v) Using your answers to (i), (iii) and (iv) or otherwise, balance the symbol equation for the reaction. Equation: SO2 + O3 → SO3 [1] (v) Using your answers to (i), (iii) and (iv) or otherwise, balance the symbol equation for the reaction. Equation: SO2 + O3 → SO3 [1] Ratio: SO2 : O3 : SO3 = 0.03 : 0.01 : 0.03 = 3 : 1 : 3 Equation: 3SO + O3 → 3SO2 balancing numbers = [1] e) Oxygen gas is prepared by the decomposition of hydrogen peroxide solution using the catalyst manganese(IV) oxygen. 2H2O2 → 2H2O + O2 A solution of hydrogen peroxide is labelled 0.1mol/dm3 (moles per litre). 25.0cm3 of this solution is decomposed completely using manganese(IV) oxide. (i) What is meant by the term catalyst? _______________________________ _______________________________ [3] (i) What is meant by the term catalyst? Substance which speeds up/increases the rate of [1] a (chemical) reaction [1] without being used up/chemically unchanged at the end [1] [3] (ii) Calculate the volume of oxygen gas produced in this decomposition. State the units. (1 mole of any gas occupies a volume of 24dm3) __________________________________ [7] (ii) Calculate the volume of oxygen gas produced in this decomposition. State the units. (1 mole of any gas occupies a volume of 24dm3) 25.0 x 0.1 Moles of H2O2 = 1000 0.0025[1] Ratio: H2O2 : O2 = 2 : 1 [1] 0.0025 [1] 0.00125[1] Moles of O2 = 2 Volume of oxygen = 0.00125 x 24 [1] = 0.03 [1]dm3 [1] (or volume of oxygen = 0.00125 x 24000 [1] = 30[1]cm3[1] 2007, Paper 1 5 Borax is a salt which is hydrated and is used in cleaning agents. The formula may be represented by Na2B4O7.xH2O. Borax dissolves in water to give a solution which acts as a weak alkali. a) 4.775g of Borax were weighed out and made up to a volume of 250cm3 with deionised water. 25.0cm3 portions of this solution were titrated against nitric acid of concentration 0.094 mol/dm3 (moles per litre). The results were recorded in the table below. Rough Titration 1st Accurate Titration 2nd Accurate Titration Initial burette reading (cm3) 0.0 0.0 0.0 Final burette reading (cm3) 26.9 26.7 26.5 Volume of nitric acid used (titre) (cm3) 26.9 26.7 26.5 Rough Titration 1st Accurate Titration 2nd Accurate Titration (i) Initial burette reading (cm3) 0.0 0.0 0.0 Final burette reading (cm3) 26.9 26.7 26.5 Volume of nitric acid used (titre) (cm3) 26.9 26.7 26.5 Calculate the average titre. _______________________________ [2] (i) Calculate the average titre. 26.2 [2]. If rough used average = 26.7 award [1] [2] (ii) The indicator used was methyl orange. State the colour change of the indicator in this titration. From ____________ to ____________ [2] (ii) The indicator used was methyl orange. State the colour change of the indicator in this titration. From orange or yellow [1] to pink or red [1] (wrong way round) = [1] [2] (iii) Calculate the number of moles of nitric acid used in this titrations. _______________________________ [2] (iii) Calculate the number of moles of nitric acid used in this titrations. Moles = 26.6 x 0.094 1000 [1] = 0.0025 [1] (2.5 x 10-3) The equation for the reaction is: Na2B4O7 + 2HNO3 + 5H2O → 2NaNO3 + 4H3BO3 (iv) Use the equation to deduce the number of moles of Borax which reacted with the nitric acid. __________________________________ _____________________________________ [2] The equation for the reaction is: Na2B4O7 + 2HNO3 + 5H2O → 2NaNO3 + 4H3BO3 (iv) Use the equation to deduce the number of moles of Borax which reacted with the nitric acid. 0.0025 1 mole borax : 2 moles nitric acid or 2 = 0.00125 [1] (1.25 x 10-3) [2] (v) Calculate the concentration of the Borax in mol/dm3 (moles per litre) _______________________________ _______________________________ [2] (v) Calculate the concentration of the Borax in mol/dm3 (moles per litre) conc = 0.00125x1000 25 [1] = 0.05 [1] mol/dm3 [2] (vi) From the mass of Borax used, calculate the concentration of Borax in g/dm3 _______________________________ [1] (vi) From the mass of Borax used, calculate the concentration of Borax in g/dm3 4.775g in 250cm3 → 4.775 x 4 = 19.1 [1] g/dm3 [1] (vii) Using your answers to parts (v) and (vi) find the formula mass of the Borax, Na2B4O7.xH2O, and hence find the value of x. (Relative atomic masses: H = 1; B = 11; O = 16; Na = 23) _______________________________ _______________________________ _______________________________ [3] (vii) Using your answers to parts (v) and (vi) find the formula mass of the Borax, Na2B4O7.xH2O, and hence find the value of x. (Relative atomic masses: H = 1; B = 11; O = 16; Na = 23) 19.1 0.05 = 382 [1] 382 = 202 [1] + 18x 18x = 180 x = 10 [1] [3] b) When Borax crystals are left in air they lose some of their water of crystallisation. To find the value of x in a sample of hydrated Borax Na2B4O7.xH2O, which had been left in air for a month, the sample was heated to constant mass. 7.28g of hydrated Borax produced 4.04g of anhydrous Borax. (Relative atomic masses: H = 1; B = 11; O = 16; Na = 23) (i) What is meant by “heated to constant mass”? _______________________________ [2] (ii) Calculate the mass of water lost. _______________________________ [1] (i) What is meant by “heated to constant mass”? heating and weighing [1] repeat until 2 readings the same [1] [2] (ii) Calculate the mass of water lost. 3.25g, 7.28 – 4.04 = 3.24g [1] (iii) Calculate the number of moles of water lost. _______________________________ _______________________________ [2] (iv) Calculate the number of moles of anhydrous Borax. _______________________________ [1] (iii) Calculate the number of moles of water lost. moles = 3.24 18 [1] = 0.18 [1] [2] (iv) Calculate the number of moles of anhydrous Borax. Moles = 4.04 202 = 0.02 [1] (v) Using your answers to (iii) and (iv) determine the value of x in Na2B4O7.xH2O. _______________________________ _______________________________ [2] (v) Using your answers to (iii) and (iv) determine the value of x in Na2B4O7.xH2O. borax : water 0.02 : 0.18 [1] 1 : 9 x = 9[1] [2] c) When anhydrous Borax is heated it decomposes according to the equation: Anhydrous Borax → Sodium metaborate + Boric Oxide Na2B4O7 → 2NaBO2 + B2O3 Calculate the mass of sodium metaborate which is produced when 5.05g of anhydrous Borax is heated. (Relative atomic masses: B = 11; O = 16; Na = 23) Calculate the mass of sodium metaborate which is produced when 5.05g of anhydrous Borax is heated. (Relative atomic masses: B = 11; O = 16; Na = 23) Moles of anhydrous borax = = 0.025 [1] Ratio 1 borax : 2 sodium metaborate 0.025 : 0.050[1] 0.05x66[1] : = 3.3 [1]g Thanks for viewing. Do consider using others in this Series of Ten. 136