TBI market - Medicortex
advertisement
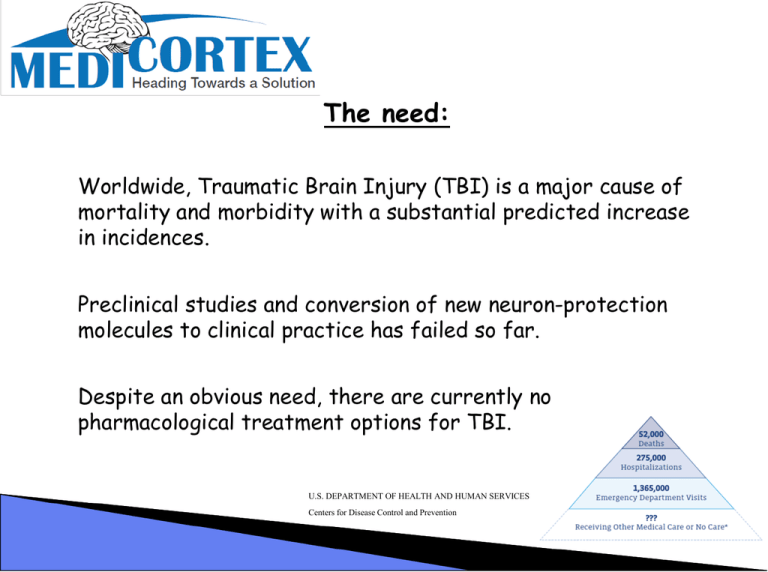
The need: Worldwide, Traumatic Brain Injury (TBI) is a major cause of mortality and morbidity with a substantial predicted increase in incidences. Preclinical studies and conversion of new neuron-protection molecules to clinical practice has failed so far. Despite an obvious need, there are currently no pharmacological treatment options for TBI. U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Disease Control and Prevention TBI is a major cause of morbidity and mortality worldwide, with an incidence of 235 per 100,000 people and a worldwide mortality of more then 1.5 million per year. The public health impact of TBI is expected to increase because the most common cause of TBI is road traffic accidents. It is projected to become the fourth leading cause of disability by 2030. In addition, TBI is a major problem in combat areas/war zone -The New England Journal of Medicine reported a study where over 15% of soldiers in Iraq are suffering from Traumatic Brian Injuries. “Pentagon estimates up to 360,000 cases of brain injury suffered by veterans of the wars in Iraq and Afghanistan (2009)”. TBI market: Currently, no effective TBI therapy exists, with patients treated through a combination of surgery, rehabilitation and pharmacological agents managing posttrauma conditions such as depression. Consequently, the estimations for global market value for TBI therapy is in the region of US$10 billion, with the majority of this accounted for by the demand across Europe and in the United States. Forecast Value of the Traumatic Brain Injury Market in the United States 2015-2020 (Assuming first products launched within the next three years. Source: Arrowhead Publishers) TBI: Complex problem- creative solution TBI is a highly complex disorder that includes varying degrees of contusion, diffuse axonal injury, hemorrhage and hypoxia. Collectively, these effects induce biochemical and metabolic changes that lead to progressive tissue damage and associated cell death. Both the early primary events and the delayed secondary alterations contribute to the resulting neurological deficits. Although preclinical studies have suggested many promising pharmacological agents, more than 30 phase III prospective clinical trials have failed to show significance for their primary endpoint. Most of these trials targeted single factors proposed to mediate secondary injury. But the complexity and diversity of secondary injury mechanisms have led to calls to target multiple delayed injury factors, either by combining agents that have complementary effects or by using multipotential drugs that modulate multiple injury mechanisms. Innovation: Innovative molecules that have a unique combination of properties were designed. In the clinic, we will not introduce one molecule with one effect but rather a combination of activities tackling several cascades using a specific compound with more then one activity. Chemistry: We have designed a bank of chemically verified molecules that can cross the blood-brain barrier (BBB), each possessing an inovative chemical spacer with two or more of the following properties for preventing secondary brain deterioration in TBI patients: • Binding of free metal ions • Anti-oxidation, • Anti-inflammation • Anti-bacterial. Work plan: 1) Chemistry, optimization of synthesis, improving yields, setting up an analytical method (HPLC), accumulating data on solubility, stability in solution and in plasma. Membrane permeability, Kd’s and LogD will be calculated. Formulations to optimize the method of treatment will be developed. 2) In-Vitro, Preliminary information of toxicity to cells will be tested (XTT, LDH). A new and innovative method for neuronal growth and monitoring single cell response to stimuli was set-up and now efficiency will be demonstrated. The LCA (liveCell array, Nunc) modified for neurons and glial cells will be recruited to demonstrate neuroprotection. 3) In-Vivo, compounds in the appropriate formulation will be tested in TBI models. A rat model of percussion injury is calibrated, tested and ready to be used in order to demonstrate efficacy. We have set-up several behavioral, memory and function tests to evaluate the outcome of the treatment. In addition we have an ElISA test optimized to measure and follow-up a marker, specific to prediction of the outcome of TBI in rats. The LCA (liveCell array™) innovative system The LCA system is used for in-vitro Proof Of Concept (POC) Other Brain Injuries: Open head Injury -Results from bullet/shred wounds. Closed Head Injury -Resulting from a slip, fall and motor vehicle crashes. Deceleration Injuries -The differential movement of the skull and the brain when the head is struck, results in diffuse axonal shearing of the brain that is slammed back and forth inside the skull. It is alternately compressed and stretched because of the gelatinous consistency. Chemical & Toxic -Also known as metabolic disorders. This occurs when harmful chemicals include insecticides, solvents, carbon monoxide poisoning, lead poisoning, etc. damage the neurons. Hypoxia (Lack of Oxygen) - When the blood flow is depleted of oxygen, even for a few minutes, then irreversible brain injury can occur from anoxia (no oxygen) or hypoxia (reduced oxygen). This condition may be caused by heart attacks, respiratory failure, drops in blood pressure and a low oxygen environment. Tumors -Tumors can cause brain injury by invading the spaces of the brain leading to direct damage. Damage can also result from pressure effects around an enlarged tumor. Surgical procedures aimed at removing the tumor may also contribute to brain injury Infections -Viruses and bacteria can cause serious and life-threatening diseases of the brain (encephalitis) and meninges (meningitis) Stroke -If blood flow is blocked through a cerebral vascular accident, cell death will result in the area deprived of blood. If there is bleeding in or over the brain (hemorrhage or hematoma) due to a tear in an artery or vein, loss of blood flow and injury to the brain tissue by the blood will also result in brain damage General awareness: General Colin L. Powell, USA (Ret.) USA grants: MediCortex USA •Medicortex is registered as an American company. •At the present, the company is being run on personal capital. •Currently, we are looking for the seed investment of $200,000 US •The next milestone will be reached after proof of concept and a demonstration of efficacy. Therefore making the company worth an estimated $2-$3 M •The use of proceeds raised is not for salary, it will be for: •1) chemical synthesis •2) in-vitro toxicology •3) in-vivo efficacy Contact: Dr. Adrian Harel Tel:+972-54-4727696 E-mail: hareladrian@yahoo.com www.medicortex.com





















