Egypt
advertisement

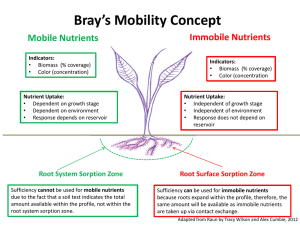
Evaluation of the Use of Synthetic Zeolite as a Backfill Material in Radioactive Waste Disposal Facility Presented by Dr Ahmed Mohamed El-Kamash Hot Lab. & Waste Management Center AEAE, Egypt Evaluate the feasibility of using synthetic zeolite NaA-X prepared from fly ash (FA) as backfill material in the proposed radioactive waste disposal facility in Egypt. Also, the migration behavior of cesium and strontium ions, as two of the most important radionuclides commonly encountered in Egyptian waste streams through the proposed backfill material is studied using mathematical models Radioactive disposal system • The principle objectives of radioactive waste management are to assure that workers and public are not harmed now or in future by the effects of radiation from the wastes and that the environment is not adversely affected. • The fundamental safety concept for the disposal of radioactive wastes is to isolate the waste from the accessible environment for a period sufficiently long to allow substantial decay of the radionuclides and to limit release of residual radionuclides into the accessible environment. A disposal system is intended to: • isolate the waste from the accessible environment for certain amount of time until waste activity reduced to acceptable hazardous level. • control the radionuclides that reach the accessible environment • limit the consequences of any unacceptable release to accessible environment Major Types of Radioactive Waste Disposal facilities: – Near surface disposal facility means a land disposal facility in which radioactive waste is disposed of in or within the upper 30 meters of the earth’s surface. – Deep Geological Disposal for high level waste such as spent nuclear fuel, >400 meters underground Repository design components • The engineering barrier system – Engineered barriers can be used as physical and /chemical obstruction to prevent or delay migration of radionuclides. • The natural barrier system – Consists of the geological media hosting the repository and any other geological formations contributing to waste isolation. Multiple barrier concept • The long term safety of a repository relies on a series of barriers : The Engineered Barrier and The natural Barrier • Multiple barrier concept is employed in which the waste form, the engineered barriers and the site itself all contribute to the isolation of the radionuclides. • The failure of one or more of these barriers will be compensated by the rest of them Function of barriers Barriers can either provide • absolute containment for a period of time, such as the metal wall of a container, or • may retard the release of radioactive materials to the environment, such as a backfill or host rock with high sorption capability. Elements of engineered barriers Backfill materials • Backfills are used for a number of purposes: void filling to avoid excessive settlement, limitation of water infiltration, sorption of radionuclides, precipitation of radionuclides. Typical materials used, either singly or as admixtures, include clays, cement grout, rock, and soil. • It is important to select the appropriate backfill. Selections of backfill materials for radioactive waste disposal have been derived from a much data on adsorption behaviour of radionuclides on several natural and synthetic materials. • For long-term performance assessment of radioactive repositories, knowledge concerning the migration of radionuclides in the backfill materials is required . • Sorption reactions are expected to retard the migration of radionuclides thereby reducing the potential radiological hazard to humans resulting from disposal of radioactive waste. In respect to fly ash Fly ash is an inorganic spherical residue obtained at coal power plants . The spherical microscopic structure of fine fly ash is related to the equilibrium between the operating forces on the molten inorganic The past applications of fly ash were restricted to its application in industry as an additive or as an adsorbent. Synthesis of zeolites from fly ash • Zeolite synthesis is one of a number of potential applications for obtaining high value industrial products from fly ash for environmental technology. • The composition similarity of fly ash to some volcanic materials, precursor of natural zeolites promoted the synthesis of zeolite from this waste material. Synthesis and characterization of pure zeolites Sorption studies Long term behavior of zeolite NaA-X blend as proposed backfill Synthesis and characterization of pure zeolites Oxide Wt % SiO2 Al2O3 Na2O MgO P2O5 SO3 Cl K2O CaO TiO2 Fe2O3 43.81 23.18 4.01 2.72 6.10 2.31 0.01 0.87 0.80 0.49 15.68 Intermediate glass content of about 66.99% I 0.0 10 20 30 40 50 60 70 2θ angle :mullite (3Al2O3.2SiO2) and : α-quartz (SiO2)] exits as crystalline substances, as identified by sharp peaks, while the presence of amorphous phases were identified by broad peaks (near 24 angle) Silica-Aumina extraction by fusion - The available silica in fly ash was extracted by the alkali fusion method using sodium hydroxide. - The amount of extracted silica was131.43g/kg fly ash. - The amount of extracted alumina was about 41.72 g/kg. Synthesis of pure NaA-X zeolite The synthesis of NaA-X zeolite blend was carried out using the molar oxide ratios of: SiO2/Al2O3 Na2O/SiO2 H2O/Na2O = 2.1 = 1.4 = 39.0 Sodium aluminate solution was used externally to adjust the SiO2/Al2O3 ratio to the desired value Flow sheet diagram for the synthesis of NaA-X zeolite blend from fly ash using extraction method Element Wt.% Na Al Si Ca Ti Mg Fe S K P other elements 27.79 33.41 38.34 0.067 0.081 0.062 <0.01 0.002 0.056 0.004 It clear that Si/Al ratio equals 1.15 which lied in the region of zeolite-A and X as reported in Breck ternary diagram <0.1 I 0.0 10 20 30 2θ angle :zeolite X and : zeolite A The spectrum exhibits fingerprint lines of both zeolite X at 2θ = 6.10 and zeolite A at 2θ = 7.20 and 9.93. 40 (a) (b) SEM (c) (d) (a) Untreated FA Smooth and spherical particle interspersed in aggregates of crystalline compounds which may correspond to α-quartz and mullite. (b) After 15 min fusion with Na OH (The amorphous aluminosilicates in fly ash were dissolved -Small surface cracks appeared - The particle surface changed, like unevenness (c) After 30 min (The surface of FA became rough and burst - Larger cracks were appeared librating small aggregates (d) After 60 min ( Small cenosphere were appeared -Several crystalline materials were precipitated onto the surface of FA particle SEM picture of the synthesized zeolite blend providing an evidence for cubic crystal characteristic for Na-A zeolite and the pyramidal octahedral crystal of Na-X zeolite Examination of Proposed backfill material: Synthetic zeolite Na A-X as backfill material in radioactive disposal facility Efficiency of the material (Capacity) Mechanical stability Test Experimental Investigations Column Studies Kinetics Studies Estimation of Sorption mechanism Equilibrium Studies Thermodynamic Models (Capacity) (Pseudo first-second order) Dispersion coefficient, DL Distr. Coeff.,Kd Chemisorption Diffusion, Di Effect of temperature, Thermodynamic Parameters, ∆H, ∆G, ∆S Long term behavior of zeolite NaA-X as backfill material in disposal facility sorption studies • Effect of pH • The effect of pH on the sorption of Cs+ ions from aqueous chloride solutions using prepared zeolite NaA-X material was investigated over the pH range from 2.0 to 8.0. • It was observed that the acidic medium has an inhibitory effect on the sorption process. This may be due to the competition behavior between hydrogen ions and studied ions for sorption onto the synthesized powder. • The uptake was continuously increased from 18.6% to 62.6% with the increase in pH value and the maximum uptake was found to be 64.1% and it was observed at pH range from 6.0 to 8.0. Sorption kinetics • Effect of time 80 qt,mg/g 60 40 20 + A higher initial removal rate within the first 30 minutes followed by slower rate till reaching plateau. Cs 2+ Sr 0 0 The amount sorbed for both ions was increased with time and attained equilibrium within 90-120min The amount sorbed of : Sr2+ > Cs+ 20 40 60 Time,min 80 100 120 Kinetic models • Pseudo first order 1.6 Y1 =1.5085-0.02336 X Y2 =1.54671-0.02787 X 1.4 k 1 log( q e q t ) log q e t 2 .303 log(qe-qt) 1.2 1.0 0.8 + Cs 2+ Sr 0.6 (Lagergren) (a) 0.4 0 10 20 30 40 Time,min • Straight line obtained suggest the applicability of the pseudo first order model to fit the experimental data over the initial stage of the sorption process up to 40 min. • Pseudo second order 3.5 3.0 Y1 =0.2174+0.02388 X Y2 =0.12195+0.02078 X t/qt,min g mg -1 2.5 t qt 2.0 1 h 1 qe 1.5 1.0 + Cs 2+ Sr (b) 0.5 (Ho and Mckay ) 0.0 0 20 40 60 80 100 120 Time,min It was shown that the sorption process of each ion follows pseudo second order model t Pseudo first and second-order rate constants for the sorption of cesium and strontium ions onto synthetic A-X zeolite blend at 298 K and 50 mg/l concentration. Metal ions First order Rate constant,k1(min-1) Second order Rate constant,k2(min-1) Cs+ 0.0537 0.0031 Sr2+ 0.0640 0.0039 Estimation of diffusion coefficient 1 2 F (t ) 1 exp( n Bt ) 2 2 n 1 n 6 1- Y =-0.13186+0.05815 X 2- Y =-0.00771+0.0587 X 2.5 2.0 1.5 Bt B 2D 1.0 + Cs 2+ Sr 0.5 i 2 r o (Boyed et al) 0.0 0 10 20 Time,min • 30 40 Metal ions Diffusion coefficient Di Cs+ Sr2+ 6.99*10-12 6.26*10-12 effective diffusion coefficient De 4.194*10-12 3.72 *10-12 In order to identify the step governing the removal rate of sorption process Sorption thermodynamics • Sorption can be described using an empirical relationship that defines the distribution of radionuclides between solid and liquid • Many isotherm models can describe sorption process such as Langmuir , Freundlch, and D-R. • The parameters of the isotherm equations express the surface properties and affinity of the sorbent, at fixed temperature and pH. Sorption of Cs+ and Sr2+ ions on zeolite NaA-X at different temperatures (Langmuir) 1600 3500 1400 Cs + Sr 2+ 3000 1200 qe(mmol/kg) qe(mmol/kg) 2500 1000 800 600 298 K 313 K 333 K 400 2000 1500 298 K 313 K 333 K 1000 500 200 0 1000 2000 3000 4000 3 Ce(mmol/m ) 5000 6000 7000 0 0 2000 4000 6000 3 Ce(mmol/m ) 8000 Sorption of Cs+ and Sr2+ ions on zeolite NaA-X at different temperatures (Freundlich) 4000 1600 1400 Cs + Sr 3500 2+ 1200 1000 qe(mmol/kg) qe(mmol/kg) 3000 800 600 298 K 313 K 333 K 400 2500 2000 298 K 313 K 333 K 1500 200 1000 0 0 1000 2000 3000 4000 3 Ce(mmol/m ) 5000 6000 7000 0 2000 4000 6000 3 Ce(mmol/m ) The metal concentration retained in the solid phase (mg/g) was calculated using the following equation : ( c 0 c e )V qe M 8000 Sorption of Cs+ and Sr2+ ions on zeolite NaA-X at different temperatures (D-R) 4000 1600 Cs 1400 + Sr 3500 2+ 1200 qe(mmol/kg) qe(mmol/kg) 3000 1000 800 600 298 K 313 K 333 K 400 200 2500 2000 298 K 313 K 333 K 1500 1000 0 0 1000 2000 3000 4000 3 Ce (mmol/m ) 5000 6000 7000 0 2000 4000 6000 3 Ce(mmol/m ) 8000 Isotherm models • Langmuir Isotherm model 3.0 5 1- Y =0.68176+6.46796E-4 X 2- Y =0.52506+6.42354E-4 X 3- Y =0.41304+6.26764E-4 X 4 2.5 Y1 =0.13334+2.889E-4 X Y2 =0.10257+2.86104E-4 X Y3 =0.07485+2.83973E-4 X 2.0 3 Ce/qe, kg/m 3 Ce/qe(m /kg) 3 2 298 K 313 K 333 K 1 1.5 1.0 0.5 298 K 313 K 333 K 0.0 0 Langmuir Sr-zeolite 0 1000 2000 3000 4000 3 5000 6000 -0.5 -2000 7000 Ce(mmol/m ) 0 2000 Langmuir cs-zeolite 4000 Ce, mmol/m o o ( C / q ) (1 / Q b ) (1 / Q ) C e e e 6000 3 8000 10000 Langmuir model parameters Table: Langmuir isotherm parameters for Cs+ and Sr2+ sorbed onto Zeolite NaA-X Metal ion Temperature (K) Q0(mmol/kg) b(L/mmol) R2 RL Cs+ 298 313 333 1546.0 1556.7 1595.6 0.948 1.223 1.517 0.995 0.995 0.996 0.123 0.098 0.087 Sr2+ 298 313 333 3461.4 3495.2 3521.5 2.166 2.789 3.793 0.997 0.998 0.997 0.042 0.031 0.024 The value of saturation capacity Q0 corresponds to the monolayer capacity Q0 and b increased with temperature showing that the sorption capacity and intensity of sorption are enhanced at higher temperatures. Isotherm models • Freundlich isotherm model 3.6 1- Y =1.91241+0.32219 X 2- Y =2.0962+0.27791 X 3.2 Y2 =2.54197+0.27235 X Y3 =2.7094+0.22861 X log qe(qe,mmol/kg) 3- Y =2.26438+0.23861 X 3.1 log qe(qe,mmol/kg) Y1 =2.41705+0.30261 X 3.5 3.0 2.9 298 K 313 K 333 K 2.8 2.7 3.4 3.3 3.2 298 K 313 K 333 K 3.1 3.0 2.0 2.2 2.4 2.6 2.8 3.0 3.2 3 log Ce(Ce,mmol/m ) Freundlich Cs-zeolite 3.4 3.6 3.8 4.0 Sr-zeolite 1.4 1.6 1.8 2.0 2.2 2.4 2.6 2.8 3 log Ce(Ce,mmol/m ) log q e log K f (1 / n ) log C e 3.0 3.2 3.4 3.6 3.8 Freundlich model parameters Table: Freundlich isotherm parameters for Cs+ and Sr2+ sorbed onto Zeolite NaA-X Kf (mmol/kg) R2 Metal ion Temperature (K) 1/n Cs+ 298 313 333 0.3222 0.2779 0.2386 81.730 124.79 183.81 0.985 0.985 0.982 Sr2+ 298 313 333 0.2681 0.2428 0.2082 342.45 416.19 573.90 0.964 0.965 0.974 1/n :value is dependent on the nature and strength of sorption process. Kf represent sorption capacity of both ions on zeolite NaA-X. Isotherm models • D-R isotherm model -6.4 1- Y =-6.01334-0.00389 X -6.6 -5.6 Y1 =-5.14828-0.00334 X Y2 =-5.19247-0.00265 X 2- Y =-6.08442-0.00293 X -5.8 3- Y =-5.97132-0.00275 X Y3 =-5.27976-0.00187 X -6.0 -7.0 ln qe(qe,mol/g) ln qe(qe,(mol/g)) -6.8 -7.2 -7.4 -7.6 298 K 313 K 333 K -7.8 200 400 2 600 (,kJ/mol) 800 Sr 1000 2+ -6.4 298 K 313 K 333 K -6.6 -6.8 -8.0 0 -6.2 -7.0 200 300 + D-R isotherm plots for sorption of Cs ions onto Zeolite NaA-X at different temperatures ln q e ln q m 400 500 2 2 600 2 (kJ /mol ) 2 700 800 900 D-R model parameters Table (6) D-R isotherm parameters for Cs+ and Sr2+ sorbed onto Zeolite NaA-X Metal ion Temperature (K) Cs+ 298 313 333 -0.00389 -0.00293 -0.00275 2445.9 2278.0 2550.9 0.966 0.963 0.958 11.337 13.063 13.480 Sr2+ 298 313 333 -0.0032 -0.0025 -0.0018 5508 5318 4934 0.986 0.988 0.988 12.50 14.00 16.62 qm(mmol/kg) R2 E(kJ/mol) qm The maximum sorption capacity , the values of the mean free energy ,E, of sorption in all cases is in the range of 8-16 k J/mol, which are within the energy ranges of ion exchange reaction Effect of Temperature In order to gain insight into the thermodynamic nature of the sorption process, several thermodynamic parameters for the present systems were calculated . G RT ln K c o ln K c 2.5 Y =7.51968-1640.95029 X ln Kc 2.0 1.5 1.0 + Cs 2+ Sr 0.5 Y =5.17944-1426.91259 X 0.0030 0.0031 0.0032 1/T K -1 0.0033 0.0034 S R H RT Thermodynamic Parameters Table (7): Values of thermodynamic parameters for sorption of Cs+ and Sr2+ions onto Zeolite NaA-X Metal Temperature Kc ΔGo ΔHo ΔSo (kJ/mol) (kJ/mol) (J/mol).K ion K Cs+ 298 1.466 -0.947 313 1.904 -1.670 333 2.420 -2.446 298 7.49 -4.988 313 9.74 -5.921 333 13.36 -7.176 Sr2+ 11.86 43.058 13.64 62.35 -The -ve values of ΔGo confirm the spontaneous nature of the sorption processes with preference towards Sr2+ than Cs + ions. - The +ve values of ΔHo for both studied ions confirms the endothermic nature of the sorption processes. - The entropy change was +ve and was greater in Sr2+>Cs+ Column investigations • Fixed bed column sorption experiments were carried out to study the sorption dynamics. The fixed bed column operation allows more efficient utilization of the sorptive capacity than batch process. • The breakthrough curves measured are useful to determine the main transport parameters under dynamic conditions. Breakthrough curves for Cs+ and Sr2+ ions sorbed onto zeolite NaA-X 1.2 1.2 2+ Flow rate=3 mL/min Bed depth=3 cm Sr 0.8 0.6 0.4 150 mg/L 100 mg/L 50 mg/L 0.2 + Flow rate=3 mL/min Bed depth=3 cm 1.0 Breakthrough, Ct/Co Breakthrough, Ct/Co 1.0 Cs 0.8 0.6 0.4 150 mg/L 100 mg/L 50 mg/L 0.2 0.0 0.0 0 200 400 600 800 Effluent Volume, mL 1000 1200 0 200 400 600 800 Effluent Volume, mL 1000 1200 Fixed Bed Data Table( 8): fixed bed data of Cs+ and Sr2+ions onto Zeolite NaA-X at different metal ions feed concentrations metal ions C0(mg/L) Cs+ Sr2+ qtot(mg) Column Bed performance% capacity(mg/g) X(mg) 50 39.0 23.35 59 23.35 100 47 28.5 55 28.5 150 58.5 30.5 52 30.5 50 57.5 37.5 65 37.5 100 75 48.75 60 48.75 150 78.75 45 57 53.5 Estimation of dispersion coefficient The dispersion coefficient may then be calculated from the breakthrough curve using the following equation dC dt C C0 c c0 1 erf 2 2 DL d C dx 2 vf dC dx L vft v L exp f erf D 2 D t L L 1 erfc 2 L v f t 2 D t L 1U 1 2 2 (UD L / v f L ) Long term behavior of the proposed backfill material (Zeolite NaA-X) in disposal facility. Transport mechanisms and governing equations • Diffusion F D dC (Fick`s law) dx • Advection-Dispersion • Radioactive decay f nvC nD C t • Sorption C qe = Kd Ce C z Modeling migration of radionuclides in the waste disposal facility System description Development of conceptual model Selection of mathematical models Selection of numerical technique Carry out simulation Performance assessment steps Conceptual model Cover Waste packages Backfill Host rock Concrete vault Simplified diagram Groundwater table Modeling migration through waste form 2 2 D C C C 2 2 t Rd x y C Where : decay constant, s-1 x : spatial coordinate in x direction x: spatial coordinate in y direction t: time, s C: contaminant concentration in the waste, Bq/ml D: diffusivity of contaminant in the waste. Rd: retardation coefficient in the waste Rate A D where A: area of the interface C w x dA Numerical solution and computer simulation 2 2 D C C C 2 2 t Rd x y C C n 1 n C i, j i, j 2 n 1 2 n 2 C n 1 2 C n C C D x i, j x i, j y i, j y i, j n C i, j 2 2 Rd 2(x) 2(y ) t x y n 1 ui, j n ui, j 2 x 2 y 1 2 R 2 n 1 R x ui, j n n n n D t Rd 2 C=u 2 n 2 n 1 x u i , j y u i , j n n u i , j u i1, j 2 u i , j u i 1, j n n u i , j u i , j 1 2 u i , j u i , j 1 2 n y u i , j Alternating Direction Implicit method (ADI) First step 1 n n 1 / 2 n 1 / 2 n 1 / 2 n n n u i 1, j u i 1, j v i , j v i , j 1 2 v i , j v i , j 1 2 u i , j R R Second step 1 n1 / 2 n1 n1 n 1 n 1 / 2 n1 / 2 2vi, j v i , j 1 2 u i , j u i 1, j u i 1, j v i , j R R Equations in Matrix form R 2 1 0 R 2 1 0 1 0 0 0 0 0 1 2 R R 1 0 0 0 0 0 1 2 R R 2 u1 u 2 u 3 u 4 u n 2 v1 v 2 v3 v 4 v n = = BCND (1) ( R 2 ) v 1 v 2 BCND ( n ) ( 2 ) v v n n 1 R BCND (1) ( R 2 ) u 1 u 2 BCND ( n ) ( 2 ) u u n n 1 R Computer program flow chart for waste model Start A Input Time = 0.0 B C Time=time *t Get values for the second row Data Yes Time >Tma x Calculate R Back substitution for the u vector No Get values in v vector top &bottom Stop Setup the coeffs. Matrices Get values in the u vector top & bottom Yes Calculate the rate M>3 No No Get values in the u vector other rows Output the rate Get values in the second row M>3 Yes Perform L.U. decomposition on v and u Coeffs. No M<3 No Get values in the B.C. Vector for u Yes Output the concentratio n profile M<3 Yes Get values in the B. C. Vector for v Calculate the concentration profile Get values in the v vector for other rows Back substitution for the v vector End Modeling migration through backfill C t n 1 / 2 2 C i, j C n i, j t n1 / 2 v x C i1, j A D 2 t 2 t 2 D xx D xx 2 x C 2D yy 2 D yy 2 n 1 / 2 i 1, j n1 / 2 C i 1, j 2D xx 2 2 C B y 2C v xx x 2 2 n 1 / 2 i, j v C y vx C n i , j1 C x yy 2 v y C vy n 1 / 2 i 1, j C y D yy 2 C n n i , j 1 D C yy 2 D F 2 C i , j C i , j 1 n1 / 2 C i , j 1 C i , j D D E C yy 2 v y v y n n Equations in Matrix form A C 0 0 A C 0 0 B 0 0 A B 0 C A B 0 0 0 0 0 C B 0 0 A B 0 C A B 0 0 0 0 0 C 0 0 0 B A 0 0 0 B A u1 u 2 u 3 u 4 u n v1 v 2 v3 v 4 v n = = D v 1 Ev 2 D v 2 Ev 3 D v 3 Ev 4 D v Ev n n 1 Fv 0 Fv 1 Fv 2 Fv n 1 D u 1 Eu 2 Fu 0 D u 2 Eu 3 Fu 1 D u 3 Eu 4 Fu 2 D u Eu Fu n 1 n n 1 Computer program flow chart for backfill model A B Initialization For the B.C. and initial condition Set up the coefficient matrices for both waste and backfill by overwriting on the diagonal and certain off diagnonal elements Get values into B.C. vector for u matrix in the waste and backfill Get values into B.C. vector for v matrix in the waste and backfill Perform L.U. decomposition on the diagonal terms for the waste and backfill If Time> Tmax A Forward substitution for the waste equations Perform convolution integral Backward substitution for the waste equations Differentiate to find concentration gradient Differentiate to find gradient Integrate and multiply by Db*Zb to find the release rate from the backfill Integrate and multiply by Dw*Z w to find the release rate from the waste Forward substitution for the backfill equations Backward substitution for the backfill to find the concentration profile due to instantaneous unit release B Output END Model validation 1 Numeriacl 0.9 Analytical 0.8 0.7 C/Co , 0.6 0.5 0.4 0.3 0.2 0.1 0 0.1 0.15 0.2 0.25 0.3 0.35 0.4 Distance, m R . x Vt C0 C ( x, t ) erfc 2 DR t 2 L exp (Ogata, 1970) Vx D L R . x Vt erfc 2 D Rt L Results of the long term studies Concentration profile of Cs in zeolite backfill after 300 y 600 500 400 C 300 200 C,Bq/m3 100 0.4 0.2 0.35 0.3 0.25 X ,m (Cs) 0.2 0.15 0.1 0.05 0 0.05 Y ,m Concentration profile of Sr in zeolite backfill after 300 y 80 70 60 50 C 40 C,Bq/m3 30 20 0.4 0.3 10 0 0.15 0.05 0.1 0.15 0.2 0.25 0.3 X ,m (Sr) 0.05 Y ,m Release rate of Cs and Sr radionuclide from the proposed zeolite backfill 1.E+01 Release Rate (GBq/Y) 1.E+00 1.E-01 1.E-02 1.E-03 1.E-04 Sr 1.E-05 Cs 1.E-06 0 50 100 150 Time (y) 200 250 300 Release rate for the Cs radionuclides from the waste form ,the proposed and commonly applied backfill 1 .E + 02 1 .E + 01 1 .E + 00 1 .E -02 1 .E -03 1 .E -04 /Y ) Waste Form (G B q Release Rate 1 .E -01 1 .E -05 Zeolite Bentonite &crushed rock 1 .E -06 0 50 100 150 Time 200 (y ) 250 300 Conclusions The results obtained in this work show the following: • The synthetic zeolite NaA-X proposed as backfill material was successfully prepared and completely characterized using XRD, XRF, and SEM techniques. • The sorption studies indicated the feasibility of using the prepared zeolite NaA-X as backfill material compared to bentonite because of its high capacity and selectivity for the concerned radionuclides (Cs and Sr) these characteristics are fundamental to the performance of such zeolite in radioactive waste interactions. conclusions • Column investigation yield a realistic picture of the sorptrion of Cs and Sr on zeolite NaA-X and lead to determination of dispersion coefficient which in turn used in migration modeling. • Transport properties of zeolite NaA-X packed column have been determined. The classical advection-dispersion model described successfully Cs and Sr breakthrough curves under saturated flow conditions. Based on this experimental data the dispersion coefficient needed for long-term migration study was determined. • The mathematical simulation performed in the long-term studies show the capability of the prepared zeolite NaA-X to prevent the migration of Cs and Sr from the repository to the environment.