The Influence of Dissolved Hydrogen on the Solubility and Transport
advertisement

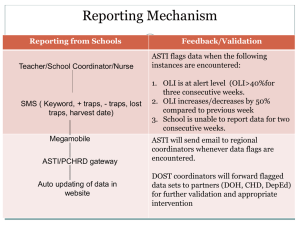
The Influence of Dissolved Hydrogen on the Solubility and Transport of Iron and Nickel in Reactor Coolant Systems OLI Simulation Conference October 23-24, 2007 Paul Sherburne Presentation Outline ► Introduction & Background Overview of Reactor Coolant System Design Basis of Reactor Coolant Chemistry Control Recent Industry Challenges • • Stress Corrosion Cracking of Vessel Penetration Nozzles Axial Offset Anomalies (Crud-Induced Power Shifts) Industry Response ► Application of OLI Model to PWR Chemistry Effects of Elevated Hydrogen on Nickel and Iron Solubility • • Model Predictions and Comparison with Literature Data Potential Impact of Elevated Hydrogen on Crud Formation ► Future Studies AREVA NP INC. OLI Simulation Conference October 23-24, 2007 2 Pressurized Water Reactor System Animated Diagram of a Pressurized Water Reactor. From the NRC Website AREVA NP INC. OLI Simulation Conference October 23-24, 2007 3 Reactor Coolant Chemistry ► Control of reactor coolant chemistry has several objectives: To minimize the general corrosion of system materials • Austenitic stainless steels (304, 316, A-286) • High-strength austenitic alloys (X-750, 718) • Nickel-base alloys (Alloys 600 & 690 and related weld materials) To maintain the integrity of the fuel rod zirconium alloy (Zircaloy) cladding To manage the exposure of personnel to out-of-core radiation fields to ALARA levels • By minimizing the generation and transport of corrosion products to the core where they become irradiated and released To moderate the nuclear reaction AREVA NP INC. OLI Simulation Conference October 23-24, 2007 4 Reactor Coolant Chemistry Parameters Controlled Parameter Concentration Range Purpose Boron 0 – ~1300 ppm Neutron Absorption ≤ 4 ppm Lithium 6.8 – 7.4 pH300°C Dissolved Hydrogen pH Control Minimize corrosion 25 – 50 cm3 (STP)/kg H2O Suppress radiolysis, establish reducing environment Temperature Range: 270°C – 330°C RC Pressure: 15.6 MPa AREVA NP INC. OLI Simulation Conference October 23-24, 2007 5 Typical Fuel Cycle AREVA NP INC. OLI Simulation Conference October 23-24, 2007 6 Current Industry Challenges ► Primary Water Stress Corrosion Cracking (PWSCC) has affected nickel-based alloys – steam generator tubing, instrumentation nozzles, pressurizer heater nozzles, and Control Rod Drive Mechanism (CRDM) penetrations since the mid-1980’s. Remedial measures have included • • • • • Increased number and frequency of inspections Repair and replacement of defective nozzles and sleeves Weld overlays of defective welds Replacement of reactor vessel heads and steam generators Injection of zinc to reduce rate of PWSCC PWSCC will continue to be a factor as plants age ► Axial Offset Anomalies (AOA) have caused axial power asymmetries in some plants having high-duty cores First observed in 1988 Has also occurred in low-duty PWRs in local areas Becoming more important as plants upgrade to higher power levels AREVA NP INC. OLI Simulation Conference October 23-24, 2007 7 Main Uses of Nickel-Base Alloys in PWRs Typical PWR Reactor Configuration AREVA NP INC. OLI Simulation Conference October 23-24, 2007 8 PWSCC in Alloys 600 and 182 of Upper Head CRDM Nozzles AREVA NP INC. OLI Simulation Conference October 23-24, 2007 9 SCC Initiation and Crack Growth Rates for Alloy 600 ► Both susceptibility to SCC and maximum crack growth rates appear to occur in proximity to the Ni/NiO phase transition Ref: Morton, et al, “Measurement of the Nickel/Nickel Oxide Transition in Ni-Cr-Fe Alloys and Updated Correlations to Quantify the Effect of Aqueous Hydrogen on Primary Water SCC,” 11th International Conference on Environmental Degradation AREVA NP INC. OLI Simulation Conference October 23-24, 2007 10 Ni/NiO Phase Transition Boundary ► Morton, et al. also conducted CER and corrosion coupon tests in deaerated water (pHt=7) to define the phase transition between Ni and NiO as a function of temperature and [H2] ► MSE model was used to determine the Ni/NiO phase boundary for comparison with these test results AREVA NP INC. OLI Simulation Conference October 23-24, 2007 Ref: Morton, et al, “Measurement of the Nickel/Nickel Oxide Transition in Ni-Cr-Fe Alloys and Updated Correlations to Quantify the Effect of Aqueous Hydrogen on Primary Water SCC,” 11th International Conference on Environmental Degradation 11 Industry Proposed Solution to Reduce CGR ► Based on the work by Morton, et al., it has been proposed that reactor coolant [H2] be increased to much higher levels to achieve slower crack propagation rates ► Potential concerns with increasing [H2] include: Effect on time to initiate cracking • Studsvik testing [Molander, et al. (2007)] suggests that decreasing [H2] delays crack initiation without significantly increasing crack growth rates Low temperature crack propagation due to increased levels of absorbed hydrogen Operational concerns with greater volume of H2 to manage Effect on Zircaloy cladding integrity • Increased H2 pickup possible hydriding Effect on corrosion product transport and deposition in highduty cores • Any impact on potential for AOA/CIPS? AREVA NP INC. OLI Simulation Conference October 23-24, 2007 12 Ni/NiO Stability (MSE Model) a – min H2 to suppress radiolysis b – present operating range c – industry proposed maximum AREVA NP INC. OLI Simulation Conference October 23-24, 2007 13 Ni/NiO Phase Transition Dependence on T and [H2] MSE Model agrees reasonably well with accepted data AREVA NP INC. OLI Simulation Conference October 23-24, 2007 14 Axial Offset Anomalies ► AOA, or Crud Induced Power Shifts (CIPS) Axial asymmetry in power observed mid-cycle in 18-24 month fuel cycles Associated with sub-cooled nucleate boiling (SNB) and substantial crud buildup in the upper part of (mostly) highduty cores Attributed to boron enrichment in fuel rod deposits (crud) • Adsorption of boron? • LiBO2 or Ni2FeBO5 precipitation? Precipitation of NiO crystals (whiskers) observed in deposits ► Mechanism for hideout of B is not clear AREVA NP INC. OLI Simulation Conference October 23-24, 2007 Ref: Bennett, et al., “Demonstration of the PWR AOA in the Halden Reactor,” Int’l Conf on Water Chemistry in Nuclear Reactor Systems, 2006 15 Application of OLI Technology ► The MSE model is being used to develop a better understanding of the effect of elevated [H2] on the transport of soluble nickel and iron species in the RCS and the precipitation of these species in the reactor core ► To achieve the best simulation of RCS chemistry, OLI Systems provided the following assistance: Improved boron-lithium chemistry at high temperatures Added boric acid & silicic acid vapor phase parameters Added several new species to the MSE database • Lithium monoborate, LiBO2 • Nickel ferrite, NiFe2O4 • Bonaccordite, Ni2FeBO5 AREVA NP INC. OLI Simulation Conference October 23-24, 2007 16 Approach ► Comparison of MSE model predictions of nickel and iron equilibrium solubilities against literature data ► Extension of model to B-Li chemistry for Ni/NiO and Fe3O4 solubility vs. pH(t) and [H2] ► Vaporization of solutions saturated in nickel and iron to simulate sub-cooled nucleate boiling (SNB) and precipitation in the upper core region ► Comparison of model predictions with physical observations AREVA NP INC. OLI Simulation Conference October 23-24, 2007 17 Model Verification Nickel Oxide Solubility Magnetite Solubility AREVA NP INC. OLI Simulation Conference October 23-24, 2007 18 Nickel Solubility at 0 cc/kg H2 AREVA NP INC. OLI Simulation Conference October 23-24, 2007 19 Nickel Solubility at 35 cc/kg H2 AREVA NP INC. OLI Simulation Conference October 23-24, 2007 20 Nickel Solubility at 70 cc/kg H2 AREVA NP INC. OLI Simulation Conference October 23-24, 2007 21 Iron Solubility at 35 cc/kg H2 AREVA NP INC. OLI Simulation Conference October 23-24, 2007 22 Iron Solubility at 70 cc/kg H2 AREVA NP INC. OLI Simulation Conference October 23-24, 2007 23 Solids Formation due to SNB for [H2] = 35 scc/kg ► Note: LiBO2 was the major precipitate beginning at a C.F. of 200. LiBO2 has been postulated to contribute to the occurrence of AOA. AREVA NP INC. OLI Simulation Conference October 23-24, 2007 24 Solids Formation due to SNB for [H2] = 70 scc/kg ► Note: precipitation of Fe3O4 and NiO is favored over NiFe2O4 at the increased dissolved hydrogen concentration AREVA NP INC. OLI Simulation Conference October 23-24, 2007 25 Effect of Steaming on Solution pH and Boiling Point Elevation AREVA NP INC. OLI Simulation Conference October 23-24, 2007 26 Observations from Modeling ► Solubility-driven precipitates on the fuel rods are nickel (metal) and magnetite. This is in general agreement with industry data. ► Increasing [H2] to 70 scc/kg from 35 scc/kg decreases the solubility of Ni metal Ni metal still exhibits retrograde solubility; however, Less nickel is available for precipitation ► Increasing [H2] increases the solubility of iron More iron is available for precipitation, which may increase porous deposit (crud) levels in the core ► At 35 scc/kg H2, steaming in porous deposits results in An increase in local alkalinity from pH(t) 7.25 to 8.5 due to the volatility of boric acid precipitation of LiBO2, NiO, NiFe2O4, and Fe3O4 ► Increasing [H2] to 70 scc/kg modifies the makeup of precipitating species Precipitation of Fe3O4 and NiO is favored over NiFe2O4 The ratio of Fe/Ni in the deposit increases ► For the cases studied, the model did not predict the formation of Ni2FeBO5 AREVA NP INC. OLI Simulation Conference October 23-24, 2007 27 Ongoing Studies ► Expand analysis to cover the complete cycle, including startup and shutdown operations ► Consider adding non-stoichiometric nickel ferrites to MSE data base ► Add silica and zinc to the existing model Identify any additional species required based on deposit analyses Model the effects of silica and zinc injection on core deposits – determine RCS silica limit ► Investigate the feasibility for modeling Zircaloy corrosion AREVA NP INC. OLI Simulation Conference October 23-24, 2007 28 Future Plans ► AREVA NP is currently working with OLI Systems to dynamically link the OLI thermodynamic engine to MatLab® to provide chemistry input to AREVA’s deposition model ► For a given chemistry (.dbs file), temperature and water analysis in MATLAB®, the thermodynamic engine determines the chemical equilibrium at the bubble point pressure and returns the results to MATLAB® ► The dynamic link to OLI has been successfully completed and test cases are being run to ensure that accuracy is maintained through the link AREVA NP INC. OLI Simulation Conference October 23-24, 2007 29 AREVA NP INC. OLI Simulation Conference October 23-24, 2007 30