Chapter 11 Thermochemistry
advertisement

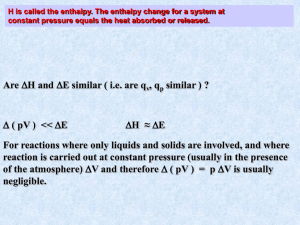
Chapter 11 Thermochemistry Objectives: • 1 Explain the relationship between energy and heat. • 2. Distinguish between heat capacity and specific heat. Thermochemistry deals with the heat changes that occur during a chemical reaction Energy Ability to do work or give heat • Heat (q) 1. energy that is transferred from one object to another because of a temp.change 2. flows from a warmer object to a cooler object • System (the universe) 1. surroundings (everything in the universe) 2. ex. Mixture of chemicals in a beaker (system) (surroundings) • Law of conservation of energy In a chemical or physical process energy is neither created nor destroyed. It can become work, stored energy or heat. • Heat flow 1. Endothermic flows into the system positive sign can feel cool 2. Exothermic heat flows out of the system negative sign can feel hot • Examples of endo/exo thermic 1. burning a piece of paper _________ 2. melting ice cubes ____________ 3. air in heated tire expands ________ 4. cooking an egg __________ • Thermochemical equations endothermic 2NaHCO3 + 129 kJ Na2CO3 + H2O + CO2 OR 2NaHCO3 Na2CO3 + H2O + CO2 ΔH = 129 kJ exothermic C3H8 + 5O2 3CO2 + 4H2O + 2043 kJ OR C3H8 + 5O2 3CO2 + 4H2O ΔH = -2043 kJ calorie quantity of heat needed to raise the temperature of 1 g of pure water 1°C 1. Calorie ( C ) - refers to food 2. calorie ( c ) – refers not to food 3. 1 Calorie = 1 kcal = 1000 cal Joule (J) – SI unit of heat and energy 1. 1 J = 0.2390 calories 2. 1 calorie = 4.184 J Ex. Convert 142,000 calories to J Specific Heat capacity or specific heat (C) 1. Amount of heat required to raise the temperature of 1 g of a substance by 1oC 2. q = C(m)(ΔT) q= heat m= mass C = specific heat ΔT = final T – initial T 4.Calculate the total amount of heat the solar pond will absorb and release on a typical day, if the mass of the water is 22,500 kg, the mass of granite is 14,500 kg. The change in temperature for both is 22.0oC. The specific heat of water is 4.184 J/goC and granite is 0.803 J/goC. Objectives: 3. Construct equations that show the heat changes for chemical and physical processes. 4. Calculate heat changes in chemical and physical processes More themrochemistry Calorimeter- equipment used to measure the amount of heat released or absorbed during a change Heat of reaction- amount of heat released or absorbed during a chemical reaction Enthalpy 1. The sum of all energy plus a term that takes into account the pressure and volume of the substance. 2. Symbol H 3. units: kJ/mole Enthalpy change (∆H) 1. ∆H = Hproducts - Hreactants 2. ∆H value + - Process heat endo absorb exo released 3. ∆H graphs C3H8 (g) + 5O2 (g)3CO2(g) + 4H2O(g) +2043 kJ/mole What can you tell from the equation? exothermic ∆H = -2043 kJ/mole heatproduct < heatreactant exothermic C (s) + H2O(g) + 113 kJ/mole --> CO(g) + H2O(g) What can you tell from the equation? endothermic ∆H = +113 kJ/mole Hproducts > Hreactants • Heat of reaction calculations 1. depend on the quantity of reactants and products 2. depend on the state of the reactants and products 3. Defined in terms of 1 mole of product Examples of enthalpy change 1. How much heat will be released if 6.0 g of carbon reacts with excess oxygen according to the equation below? C(s) + O2 (g) --> CO2 (g) ∆H = -394 kJ/mole Using limiting reagents How much heat will be transferred when 0.50 mol of TiO2 reacts with 1.60 mol of HCl according to the following equation? TiO2(s) + 4HCl(g) -->TiCl4(l) + 2H2O(g) ∆H=-67kJ/mole • Heat of combustion 1. The heat released by the complete combustion of 1 mole of a substance 2. Defined in terms of 1 mole of reactant 3. Ex. How much heat will be released if 1.0 g of hydrogen peroxide (H2O2) decomposes? 2H2O2 (l) 2H2O (g) + O2 ΔH= -190 kJ/mole Calorimetry 1. The accurate and precise measurement of heat change for chemical and physical processes 2. qrxn = -qH2O The negative means the opposite sign for the system 4. Example: A 13.7 g sample of Pb(NO3)2 reacts with 85.0 g of water. The temperature drops from 23.4oC to 19.7oC. Find the ∆H. Pb(NO3)2 --> Pb+2 + 2 NO3- Objectives: • 5. Apply Hess’s Law of heat summation to find heat changes for chemcial and physical processes • 6. Calculate heat changes using standard heats of formation. Hess’s Law 1. The summation of theoretical equations and the enthalpy 2. ∆Hnet = ∆H1 + ∆H2 3. Applying Hess’s Law Example S(s) + O2(g) --> SO2(g) ∆H = -297 kJ 2SO3(g) --> 2SO2(g) + O2(g) ∆H = 198 kJ Answer: 2S(s) + 3O2(g) --> 2SO3(g) ∆H = ? Example 2: H2S(g) + 3/2 O2(g) -->H2O(l) + SO2 ∆H = -563 kJ CS2(l) + 3O2(g) --> CO2(g) + 2SO2(g) ∆H = -1075 kJ CS2(l) + 2H2O(l) --> CO2(g) + 2H2S(g) ∆H = ?