Nature Chem. 2012
advertisement
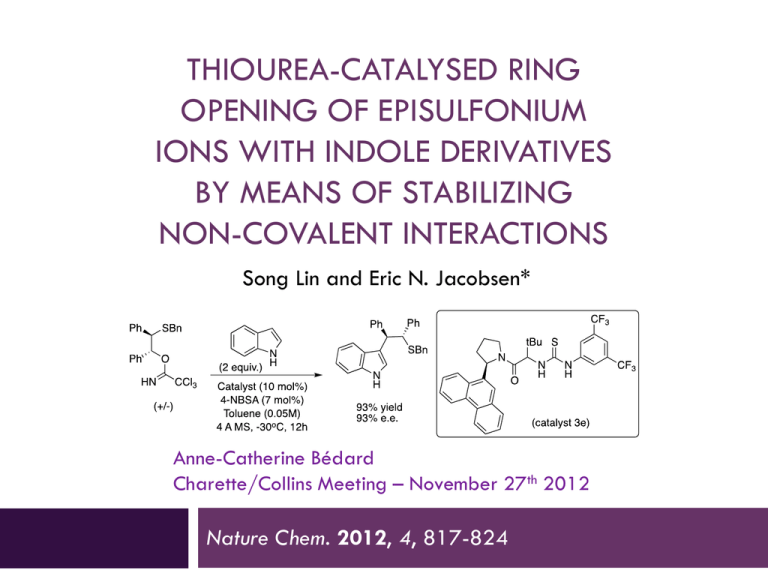
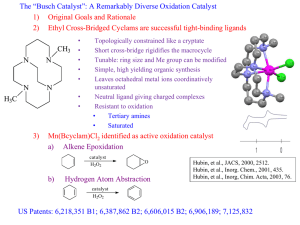
THIOUREA-CATALYSED RING OPENING OF EPISULFONIUM IONS WITH INDOLE DERIVATIVES BY MEANS OF STABILIZING NON-COVALENT INTERACTIONS Song Lin and Eric N. Jacobsen* Anne-Catherine Bédard Charette/Collins Meeting – November 27th 2012 Nature Chem. 2012, 4, 817-824 Discovery 2 Urea were originally designed as chiral ligand for Lewis acidic metal The observation of enatioselectivity in the absence of the metal was unanticipated ! M. S. Sigman, E. N. Jacobsen, J. Am. Chem. Soc. 1998, 120, 4901-4902. M.S. Sigman, P. Vachal, E.N. Jacobsen, Angew. Chem. Int. Ed. 2000, 39, 1279 – 1281 Taylor, M. S., Jacobsen, E. N. Angew. Chem. Int. Ed. 2006, 45, 1520-1543. Lewis vs Brønsted Acid Catalysis 3 “Why did the report of Yates and Eaton, and not that of Wasserman, capture the imagination of the early practitioners of asymmetric catalysis, leading to the current situation where chiral Lewis acid catalysis, rather than chiral Brønsted acid catalysis, is the dominant strategy for the promotion of enantioselective additions to electrophiles ?” Taylor, M. S. and Jacobsen, E. N. Yates, P., Eaton, P. J. Am. Chem. Soc. 1960, 82, 4436-4437. Wassermann, A. J. Chem. Soc. 1942, 618-621. Taylor, M. S., Jacobsen, E. N. Angew. Chem. Int. Ed. 2006, 45, 1520-1543. H-Bonding Catalysis in Enzymes 4 Lewis vs Bronsted Acid Non-covalent catalysis via H-Bonding Mimic the mode of action of enzymes by design of small molecule Ex : Serine protease 16 to 30 kDa Zhang, Z. G., Schreiner, P. R. Chem. Soc. Rev. 2009, 38, 1187–1198. Enzyme vs Small Molecule Catalysis 5 Enzymes : Accelerate reactions and impart selectivity as they stabilize specific transition structures through networks of cooperative interactions Chiral small-molecule : Catalysts is rationalized typically by the steric destabilization of all but one dominant pathway. However, stabilizing effects also play an important role in small-molecule catalysis (rare mechanistic characterization) Lin, S., Jacobsen, E. N. Nature Chem. 2012, 4, 817-824 Proposal 6 Thiourea : suitable host for an episulfonium ion formed in situ through interactions with the chiral counteranion Friedel–Crafts-type indole alkylation reaction Search for the Episulfonium Ion 7 Non-nucleophilic leaving group was required to achieve the desired reactivity Otherwise major product is addition of chlorine atom. Hamilton, G. L., Kanai, T. & Toste, F. D. J. Am. Chem. Soc. 2008, 130, 14984–14986. Optimization - Acid 8 Entry Acid Yield (%) e.e. (%) 1 HCl 10 5 2 HOTf 73 32 3 FSO3H 78 19 4 2,4-diNBSA 79 63 5 4-NBSA 72 73 6 4-NBSA (w/o cat.) 7 n/a Need a non-nucleophillic anion for the acid (entry 1 major product is Cl addition) Sulfonate group work better/strong counterion effect Optimization – Catalyst 9 Entry Catalyst Yield (%) e.e. (%) 1 A (Ar = H) 16 12 2 B 72 73 3 C 84 84 4 D 80 85 5 E 93 93 6 F 91 91 7 G 97 88 8 4E (urea) 98 92 No direct correlation between size of the aromatic group and e.e. (best = phenantryl) No direct interaction of the thiourea sulfur atom (Lewis based catalysis) Scope – Leaving Group 10 Entry Leaving group Yield (%) e.e. (%) 1 2 3 87 91 85 87 85 85 Choice of leaving group doesn’t have an effect on the enantioselectivity 1st step is protonation of trichloroacetamide Substrate Scope – Mecanism Insight 11 Entry R group Yield (%) e.e. (%) 1 Bn 99 93 2 Ph 84 80 3 4-F-C6H4 73 81 4 4-Me-C6H4 76 87 5 2-naphtyl 90 88 6 PMB >99 94 7 Me 72 84 8 tBu 89 87 Benzyl is better than phenyl and alkyl Rational 12 DFT : Benzylic protons in S-Benzyl episulfonium ions partial positive charge enhance attractive interactions with the catalyst Substrate Scope – Indole Substitution 13 Entry Substituant Yield (%) e.e. (%) 1 5-Me 97 91 2 5-OMe 93 93 3 5-Br 83 92 4 5-F 88 95 5 6-F 92 85 6 4-OMe 83 91 7 2-Me 95 79 8 N-Me 54 3 9 Benzotriazole 92 80 Indole N-H motif may be involved in a key interaction during e.e.-determining transition state Substate Scope - Episulfonium Substitution 14 Entry Substitution Yield (%) e.e. (%) 1 3-MeO-C6H4 85 93 2 3-F-C6H4 97 95 3 3-Me-C6H4 95 93 4 2-Me-C6H4 99 79 5 4-F-C6H4 91 45 6 4-Me-C6H4 89 60 7 4-MeO-C6H4 67 6 8 4-CF3-C6H4 18 5 9 -(CH2)4- 16 9 10 - 87 87 Para substitution decreases the enantioselectivity Interaction of the C-H with thiourea-bond sulfonate? Proposed Mechanism 15 1. Protonation of trichloroacetamide 2. Formation of episulfonium ion (endothermic ionisation) 3. Nucleophillic attack 4. Rearomatisation Kinetic Studies - in situ IR 16 Rate accelerated by chiral thiourea vs 4-NBSA alone 2.0±0.1 kcal/mol 0th order in substrate and 1st order in 4-NBSA Quantitative protonation before rds pKa 4-NBSA ≈ -7 and pKa substrate ≈ 2 1st order in indole (present at rds) Episulfonium-4-NBSA (covalent adduct) is the resting state of the substrate Denmark, S. E.; Vogler, T. Chem. Eur. J. 2009, 15, 11737-11745. Proposed Mechanism 17 1. Protonation of trichloroacetamide 2. Formation of episulfonium ion (endothermic ionisation) 3. Nucleophillic attack 4. Rearomatisation 5-Substituted Indole : Rate Comparison 18 Catalysed by 4-NBSA Catalysed by 4-NBSA and thiourea Better nucleophile = faster rate Consistent with addition being rds! No KIE when 3-D-indole is used (0.93±0.12); if rearomatisation was rds kH/kD >2.5 Proposed Mechanism 19 1. Protonation of trichloroacetamide 2. Formation of episulfonium ion (endothermic ionisation) 3. Nucleophillic attack 4. Rearomatisation Catalyst-Substrate Interactions NMR Studies 20 NMR showed attractive interactions between the aromatic group in 3e and a-protons in 5 Shift (downfield) observed for the 2 N-H in thiourea : consistent with H-Bond Kelly, T. R.; Kim, M. H. J. Am. Chem. Soc. 1994, 116, 7072-7080. Xu, H.; Zuend, S. J.; Woll, M. G.; Tao, Y.; Jacobsen, E. N. Science 2010, 327, 986-990. Indole Structure N-H is important for high yield and e.e. pKa indole rate Rate is correlated with nucleophilicity and H-bond donor properties H-Bonding with Thiourea 22 Aromatic Group on Thiourea 23 The arene affect may be caused by (1) acceleration of the major pathway through transition-state stabilization (2) inhibition of pathways that lead to the minor enantiomer through destabilizing interactions. Enantioselectivity increases because variations of the aryl component of the catalyst 3 are, indeed, tied to stabilization of the major transition structure Uyeda, C. & Jacobsen, E. N. J. Am. Chem. Soc. 2011, 133, 5062–5075 Proposed Model for Enantioselection 24 Conclusion 25 Enantioselective reaction : addition of indole to the episulfonium ion Rate acceleration/enantioselectivity by thiourea catalyst attractive non-covalent interactions in TS stabilized by anion binding of the thiourea to the sulfonate general base activation of the indole via a catalyst amide–indole N–H interaction cation-p interaction between the arene of the catalyst and the benzylic protons of the episulfonium ion “We anticipate that characterization of these enzyme-like noncovalent stabilizing elements with small-molecule catalysts such as 3e may enable the future design and application of such biomimetic strategies in organic asymmetric synthesis.” Lin, S.; Jacobsen, E. N. Nature Chem. 2012, 4, 817-824 Enzyme-Like Non-Covalent Stabilizing Elements : New Concept ? 26 Xu, H., Zuend, S. J., Woll, M. G., Tao, Y. & Jacobsen, E. N. Science 2010, 327, 986–990. Uyeda, C. & Jacobsen, E. N. J. Am. Chem. Soc. 2011, 133, 5062–5075. Thiourea Synthesis 27 Different Types of H-Bonding Interactions 28 What’s a Good H-Bond Donor ? 29 pKa propency to dimerize mode of activation urea 26 high Double H-Bond thiourea 20 low Double H-Bond bisphenol 18 low Double H-Bond guanidinium 15 low Double H-Bond diol 20 - Single H-Bond Connon, S. J. Chem. Eur. J. 2006 , 12, 5418-5427. Taylor, M. S.; Jacobsen, E. N. Angew. Chem. Int. Ed. 2006 , 45, 1520-1543. Doyle, A. G.; Jacobsen, E. N. Chem. Rev. 2007 , 107 , 5713-5743. Akiyama, T. Chem. Rev. 2007 , 107 , 5744-5758. Substrate Synthesis 30 Catalyst Investigation 31 pKa Corrected 32 Catalyst Investigation 33 Use of a Chiral Phosphoric Acid 34