An Unsaturated Nickel(0) NHC Catalyst: Facile
Preparation and
Structure of Ni(0)(NHC)2, Featuring a Reduction Process
from
Ni(II)(NHC)(acac)2
Organometallics 2008, 27, 6020–6024
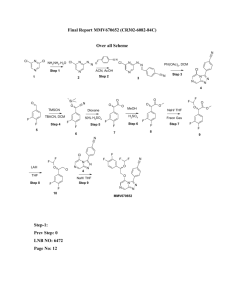
Synthesis of Ni(NHC)(acac)2 (1a and 1b).
In a typical example,
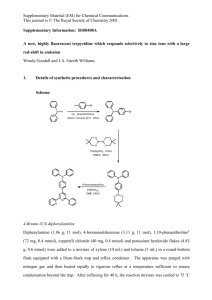
bis(2,6-diisopropylphenyl)imidazolium chloride (468 mg, 1.10
mmol), NaOtBu (115 mg, 1.20 mmol), and THF (15 mL) were
added to a 50 mL flask and stirred at room temperature for 30 min,
followed by addition of Ni(acac)2 (257 mg, 1.00 mmol) in THF (5
mL). After stirring for 2 h, the resulting suspension was filtered
with Celite and evaporated. Wash with with hexane and drying
under reduced pressure gave a pale green solid (536 mg, 83%) as
1a. Anal.
Synthesis of Ni(NHC)2 (2a) from 1a. The complex 1a (129
mg, 0.20 mmol), bis(2,4,6-trimethylphenyl)imidazolium chloride
(85.0 mg, 0.20 mmol), NaH (96.0 mg, 4.00 mmol), and 1,4-dioxane
(15 mL) were added to a 50 mL flask and stirred at 100 °C for
12 h. After the volatile solvent was removed under reduced pressure,
the residual solid was extracted with hexane. The resulting
suspension was filtered through Celite and evaporated to give a
deep purple crystalline solid containing 2a and IPr carbene.
Recrystallization from hexane at -30 °C afforded 2a in 45% yield
(74.7 mg)
Synthesis and Structures of Nickel Halide Complexes Bearing
Mono- and Bis-coordinated N-Heterocyclic Carbene Ligands,
Catalyzing Grignard Cross-Coupling Reactions
Organometallics 2006, 25, 3422-3427
Preparation of 1a, 1b, 2a, and 2b. The general procedure for
preparation of 1a, 1b, 2a, and 2b is as follows. In a typical example,
bis(2,6-diisopropylphenyl)imidazolium chloride (117.0 mg, 0.270
mmol) and THF (1.5 mL) were added to a 20 mL Schlenk tube
and stirred for 15 min, followed by addition of KOtBu (36.7 mg,
0.327 mmol) and stirring for 20 min. The resulting pale yellow
suspension was filtered through a small amount of Celite and added
to a THF solution of [NiCl2(PPh3)2] (73.5 mg, 0.112 mmol) with
vigorous stirring. The solution was then stirred for 10 min, and the
volatile reagents were removed under reduced pressure. After
extracting with hexane, sublimation of the evaporated residue at
80-110 °C for 1 h removed the triphenylphosphine, giving 2a (81.4
mg, 0.089 mmol, 80% yield). No contaminating phosphine was
confirmed on the basis of 1H and 31P NMR spectroscopy.
First Chelated Chiral N-Heterocyclic
Bis-Carbene Complexes
ORGANIC LETTERS 2000 Vol. 2, No. 8 1125-1128
Trans-diiodo-[1,1′-(2,2′-dimethyl-1,1′-binaphthyl)-3,3′dimethyldiimidazoline-2,2′-diylidene] nickel (II) (2a)
(a) Preparation in hot NMP Imidazolium salt 7 (29 mg, 0.041mmol) and
nickel acetoacetate (21 mg, 0.082 mmol) were stirred in NMP (0.20 mL)
at 200 °C for 1 h. The solvent was removed by distillation under high
vacuum, and the residue was dissolved in CH2Cl2. Chromatography of
the residue with CH2Cl2 as eluant gave complex 2a (7.9 mg, 26%) as a
red solid.
(b) Preparation from pre-formed carbene at rt Imidazolium salt 7 (15 mg,
0.022 mmol) and potassium t-butoxide (6.0 mg, 0.053 mmol) were
stirred in THF (8 mL) at rt under N2 for 10 minutes.
Bis(triphenylphosphine)nickel chloride (17 mg, 0.026 mmol) was added
in THF (2mL) and the reaction was stirred for 19 hours and then
concentrated. Column chromatography of the resultant residue with
CH2Cl2 as eluant gave complex 2a (7.4 mg,45%) as a red solid.