Carbohydrate acetals and ketals
advertisement
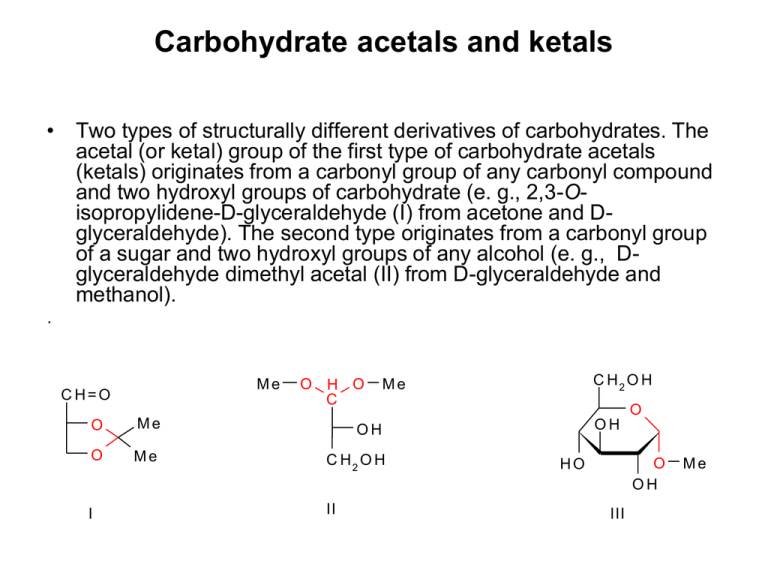
Carbohydrate acetals and ketals • Two types of structurally different derivatives of carbohydrates. The acetal (or ketal) group of the first type of carbohydrate acetals (ketals) originates from a carbonyl group of any carbonyl compound and two hydroxyl groups of carbohydrate (e. g., 2,3-Oisopropylidene-D-glyceraldehyde (I) from acetone and Dglyceraldehyde). The second type originates from a carbonyl group of a sugar and two hydroxyl groups of any alcohol (e. g., Dglyceraldehyde dimethyl acetal (II) from D-glyceraldehyde and methanol). . Me C H=O O H O C O Me OH O Me C H2 O H I II C H2 O H Me O OH O OH HO III Me Carbohydrate acetals and ketals O C H2O H O OH O OH O OH HO III Me OH OH IV Also glycosides are sugar acetals (derived from aldoses) or ketals (derived from ketoses), e. g., methyl α-D-glucopyranoside (III), but also internal glycosides, e. g., 1,6-anhydro--D-glucopyranose (IV) (red colour shows the acetal functional group). Carbohydrate acetals and ketals • Acetone, benzaldehyde, acetaldehyde and formaldehyde are most often employed carbonyl compounds for preparation of ketals and acetals of the first type. • According to these starting carbonyl compounds, they are called as isopropylidene ketals (1,2;5,6-di-Oisopropylidene--D-glucofuranose, 1,2-O-isopropylidene-D-glucofuranose, 1,2;3,4-di-O-isopropylidene--Dgalactopyranose), and benzylidene (4,6-O-benzylidene-Dglucopyranose), ethylidene and methylene acetals of carbohydrates. 1,2:5,6-di-O-isopropylidene--D-glucofuranose (I) (obsolete name, diacetone glucose) – crystalline compound, m. p. 110 °C, []D -180° (water), soluble in water and many organic solvents. The acid hydrolysis rate of its 5,6-O-isopropylidene group is 40-times higher than that in position 1,2. This is employed for preparation of another important derivative, 1,2-O-isopropylidene-D-glucofuranose (II). Ketal I is employed as starting compound in many syntheses. Thus, e. g., the intermediates, obtained either after oxidation of its free hydroxyl group to 3-oxo derivative, or after its Osubstitution, are employed for preparation of aminosaccharides, deoxysaccharides or branched-chain saccharides. C H2 O H (C H 3 ) 2 C O OH OH HO OH C H3C OC H3 O C H2 O C H2 O H O + H OH Z nC l 2 a le b o H2S O4 O O I HO O OH H2 O O O C (C H 3 ) 2 II C (C H 3 ) 2 1,2;3,4-di-O-isopropylidene--D-galactopyranose • Is employed in synthesis of saccharides and their derivatives, e. g., D-fucose (6-deoxy-D-galactose) or D-galacturonic acid. C H2 O H HO C H2 O H O OH OH OH C H3C OC H3 Z nC l 2 a le b o H2S O4 O COOH O NOx O O O O O O a le b o TEMPO O O TsC l, P y C H 2 O Ts O O C H3 LiA lH 4 O O O O O O O O • Carbohydrate acetals and ketals are stable in basic and neutral solutions. In acid solutions they decompose to the starting sugar and carbonyl compound. Their hydrolysis rate is highest for benzylidene acetals and decreases in the order isopropylidene ketals, ethylidene acetals and methylene acetals. From carbohydrate benzylidene acetals, the saccharide can be regenerated also by hydrogenolysis on paladium, similarly as from benzyl ethers. C H3 O C H3 O H 3O + O HO O OH OH O O OH D-fucose (6-deoxy-D-galactose) 4,6-O-benzylidene-D-glucopyranose (I) C H2 O H O O OH OH HO O Ph C H O O Ph OH OH Zn Cl 2 O O Ph OH O O OH OH I O II 40 % of 4,6-O-benzylidene-D-glucopyranose (I) can be isolated by reacting D-glucose with 1 mol of benzaldehyde. An excess of benzaldehyde gives rise to 1,2:4,6-di-O-benzylidene-α-D-glucopyranose(II). Ph C H2 O H (C H 3 ) 2 C O OH OH (C H 3 ) 2 C ( O C H 3 ) 2 O C H2 O O OH HOTs HO O D M F a le b o D M E OH O C H2 O H O P hC H ( O M e ) 2 O OH HO C (C H 3 ) 2 OMe OH O Ph OH HOTs D M F a le b o D M E O OMe OH Nowadays, more modern, transacetalization (transketalization) reagents are employed for preparation of carbohydrate acetals and ketals; acetone dimethyl ketal instead of acetone and benzaldehyde dimethyl acetal instead of benzaldehyde. Conformational analysis of the carbohydrate ketals and acetals Ketones (R1-CO-R2), reacting with hydroxyl groups of carbohydrates, preferentially provide the termodynamically more favourable five-membered cyclic ketals of the 1,3-dioxolane type. The characteristic examples are Oisopropylidene ketals (R1 = R2 = Me). 1 carbohydrate moiety 2 The reason is that both the bulky substituents R1, R2 are placed in equivalent, degenerated quasiequatorial (or quasi-axial) positions. carbohydrate moiety Aldehydes (R-CH=O), reacting with hydroxyl groups of carbohydrates, preferentially provide the termodynamically more favourable six-membered cyclic ketals of the 1,3-dioxane type. The characteristic examples are Obenzylidene acetals (R = Ph). In this case, the bulky substituent R is placed in equatorial position and hydrogen atom in the axial position. Isopropylidene ketals of common aldohexoses C H2 O H (C H 3 ) 2 C O OH OH HO OH D -g lu kó za C H3C O C H3 O C H2 O OH Z nC l2 a le b o H2S O4 O Me O O O O Me Me O O HO O Me C (C H 3 ) 2 O C H2 O H (C H 3 ) 2 C O O HH O OH HO D -m a n ó za C H3C O C H3 O C H2 O O O Z nC l2 a le b o H2S O4 OH OH D -g a la któ za OH Me C H2 O H O OH O C H3C O C H3 Z nC l2 a le b o H2S O4 O OH O C (C H 3 ) 2 C H2 O H HO O Me O O Me Me OH O O O O O (C H 3 ) 2 C O C (C H 3 ) 2 Me Me O O O Me Me O C H2 O H (C H 3 ) 2 C O O HH O HO (C H 3 ) 2 C (O C H 3 ) 2 OH O C H2 O O Me O HOTs D M F a le b o D M E O Me OMe O O OMe C (C H 3 ) 2 O O Me Me D -m a n ó za In case of employing more modern transketalization reagent, acetone dimethyl ketal instead of acetone, D-mannose does not afford 1,2;5,6-di-O-isopropylidene--D-mannofuranose, but its glycoside, methyl 1,2;5,6-di-O-isopropylidene--D-mannofuranoside. Acetals and ketals of alditols OH OH OH O PhC H O HO OH + OH Ph Ph HO H O O O Ph Ph O O Ph O O O O OH OH O OH OH O Ph OH D-glucitol 2,4-Obenzylidene-D-glucitol (sorbitol) OH HO 1,3:2,4-di-Obenzylidene-D-glucitol 1,3:2,4:5,6-tri-Obenzylidene-D-glucitol 1,2:5,6-di-O-izopropylidén-D-manitol C H 3C O C H 3 HO OH OH OH D-manitol k ys e lin a (rôzne podmienky) alebo 1,2:3,4:5,6-tri-O-izopropylidén-Dmanitol Acetals and ketals of alditols OH OH OH O PhC H O HO OH + OH Ph Ph HO H O O O Ph Ph O O Ph O O O O OH OH O OH OH O Ph OH D-glucitol 1,3:2,4:5,6-tri-Obenzylidene-D-glucitol (sorbitol) OH HO 1,2:5,6-di-O-isopropylidene-D-mannitol C H 3C O C H 3 HO OH OH OH D-mannitol k ys e lin a Acid (rôzne (different podmienky) condition) or 1,2:3,4:5,6-tri-O-isopropylidene-D-mannitol Synthetic employment of carbohydrate acetals and ketals OH OH O O Ph HO N aIO 4 O OH + OH H Ph HO H2O O OH O HO HO H2 O OH OH O HO O OH OH 2,4-O-benzylideneD-glucitol O CH2 O L-xylose O CH2 O CH2 O O O NaBH4 PDC OH O O 1,2;5,6-di-O-isopropylidene-D-glucofuranose O O + H O O O O HO H 2O D-allose O 1,2;5,6-di-O-isopropylidene-D-allofuranose Free sugars can be practically released from all sugar acetals or ketals by hydrolysis with a 3 N strong acid at room temperature within 48 hours.