M5_Chapter4
advertisement

CHAPTER 4
ENVIRONMENTAL FATE
Introduction
This chapter serves as a basis to identify the hazards
associated with different substances used and
produced in the chemical process, including raw
materials, products and or byproducts.
It would then be possible to do follow up with an
exposure assessment and a dose-response
assessment which are needed to perform risk
characterization
Substance Classification Tree
What Substances?
Physical + Chemical
Properties
Old Analyses
Estimating Exposure
And
Environmental Effects
Classifying the
Substances based on risk
Performing P2 on the
substances...
Chemical Properties Used to Perform
Environmental Risk Screenings
Environmental Process
Relevant Properties
Dispersion and Fate
Volatility, density, melting point,
water solubility, effectiveness of
waste, water treatment.
Persistence in the
Environment
Atmospheric oxidation rate,
aqueous hydrolysis rate, photolysis
rate, rate of microbial degradation,
and adsorption.
Continued on the following slide
Chemical Properties Used to Perform Environmental Risk Screenings
Environmental Process
Relevant Properties
Uptake by Organisms
Volatility, Lipophilicity,
Molecular Size,
Degradation Rate in Organism.
Human Uptake
Transport Across Dermal Layers,
Transport Rates Across Lung
Membrane, Degradation Rates
within the Human Body.
Toxicity and other
Health Effects
Dose-Response Relationships.
Boiling Point
• Distinguishes gas and liquid partitioning
• Using the substance’s structure, it can be estimated by :
Tb= 198.2 + Σ nigi
(4.1)
Where:
Tb: normal boiling point (at 1 atm) (K)
ni : number of groups of type i in the molecule,
gi : contribution of each functional group to the boiling point
Corrected using :
Tb (corrected) = Tb – 94.84 + 0.5577*Tb + 0.0007705*(Tb)2
(Tb 700K)
(4.2)
Tb (corrected) = Tb + 282.7 – 0.5209*Tb
(Tb > 700K)
(4.3)
Example : Boiling Point Estimation
Estimate the Normal Boiling Point for diethyl ether.
Diethyl ether has the molecular structure CH3-CH2-O-CH2-CH3
Solving :
Group
-O2(-CH3)
2(-CH2)
gi contribution
25.16
2(21.98)
2(24.22)
The actual boiling point for diethyl ether is 307.65 K
Example : Boiling Point Estimation (Continued)
a) Using equation 4.1 :
Tb (K)= 198.2 + Σ nigi
Tb (K)= 198.2 + 2(21.98) + 2(24.22) + 25.16
Tb = 315.76
b) Using equation 4.2 :
Tb (corrected) = Tb – 94.84 + 0.5577*Tb - 0.0007705*(Tb)2
Tb (corr) = 315.76 – 94.84 + 0.5577(315.76) - 0.0007705(315.76)2
Tb (corrected) = 320.2 K
Melting Point
• Distinguishes solid and liquid partitioning.
• Can be estimated using the substance’s boiling point :
Tm (K) = 0.5839 * Tb (K)
Where :
Tm : Melting Point in Kelvins.
Tb : Boiling Point in Kelvins.
(4.4)
Example : Melting Point Estimation
Estimate the Melting Point for diethyl ether.
Solving :
Using equation 4.4 to calculate the Tm :
Tm (K) = 0.5839 * Tb (K)
Tm (K) = 0.5839 * 307.65 K
Tm = 179.634 K
Vapor Pressure
• Higher Vapor Pressure = Higher Air Concentrations
• Can be estimated using the following equations :
ln Pvp = A + B/(T - C)
(4.5)
Where : T = Tb at 1 atm
ln(1 atm) = 0 = A + B/(Tb – C)
(4.6)
ln Pvp(atm) ={[A(Tb – C)2] / [0.97*R*Tb]}*{1/(Tb – C)-1/(T – C)}
(4.7)
the parameters A and C can be estimated using :
C = -18 + 0.19 Tb
(4.7a)
A = KF*(8.75+ R ln Tb)
(4.7b)
Vapor Pressure (continued)
For solids :
ln P = -(4.4 + lnTb) * {1.803*[(Tb/T)- 1)] - [0.803*ln (Tb/T)]}
- 6.8(Tm/T-1) (4.8)
Where :
Pvp : vaporization pressure (atm).
T : absolute temperature and Tb is the boiling point at 1 atm.
A and C are empirical constants.
B : a parameter related to the heat of vaporization.
KF : a correction factor.
R : gas constant ; 1.987 L-atm K-1 mol-1
Tm : melting point (K).
Example : Vapor Pressure Estimation
Estimate the Vapor Pressure for diethyl ether
Using the predicted value of 315.76 K:
C = -18 + 0.19Tb = -18 + 0.19(320.2) = 41.9944
A = Kf (8.75 + R ln Tb) = 1.06 [8.75 + 1.987 ln(320.2)] = 21.3962
(4.7.a)
(4.7.b)
(4.7)
ln Pvp = {[A(Tb – C)2] / [0.97*R*Tb]}*{1/(Tb – C) - 1/(T – C)}
= {[21.39(315.76-41.99)2] / [0.97(1.987)(315.76)]}*{1/(273.76) – 1/(256)}
Ln Pvp = -0.6677; Pvp = 0.5128 atm = 389.79 mm Hg.
Repeating the calculation for the experimental boiling point leads to a vapor
pressure estimated of Pvp = 0.6974 atm = 530.06 mm Hg.
Octanol-Water Partition Coefficient
• Describes partition between an aqueous phase and it’s
suspended organic phases.
• Can be estimated using the substance’s structure :
log Kow = 0.229 + Σ nifi
(4.9)
log Kow (corrected) = 0.229 + Σ nifi + Σ njcj
(4.10)
Where:
Kow : Octanol-Water Partition Coefficient.
ni : number of groups i in the compound.
fi : factor associated with the group i
nj : number of groups j in the compound that have correction
factors.
cj : correction factor for each group j
Example : Octanol-Water Partition
Coefficient Estimation
Estimate the Octanol-Water Partition Coefficient for diethyl ether.
Solving :
Group
-O2(-CH3)
2(-CH2)
fi contribution
-1.2566
2(0.5473)
2(0.4911)
Using equation 4.9 : log Kow = 0.229 + Σ nifi
log Kow = 0.229 + 2(0.5473) + 2(0.4911) + (1.2566)
log Kow = 1.0492 ≈ 1.05
therefore
Kow = 11.2
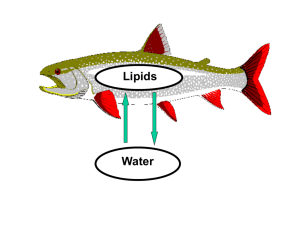
Bioconcentration Factor
• Describes partitioning between aqueous and lipid phases
in living organisms.
• Higher bioconcentration factors = higher quantity of
bioaccumulation in living organisms
• Can be calculated using :
log BCF = 0.79*(log Kow) – 0.40
(4.11)
log BCF = 0.77*(log Kow) – 0.70 + Σ jj
(4.12)
Where :
BCF : Bioconcentration Factor.
Kow : octanol-water partition coefficient.
jj : correction factor for each group.
Example : Bioconcentration Factor
(BCF) Estimation
Estimate the Bioconcentration Factor for diethyl ether.
Solving :
Using equation 4.9 we obtain log Kow :
log Kow = 0.229 + Σ nifi
log Kow = 1.0492 ≈ 1.05
Using equation 4.11 we can calculate BCF :
log BCF = 0.79*(log Kow) – 0.40
log BCF = 0.79* (1.05) – 0.40
log BCF = 0.4295
therfore
BCF = 2.6884
Water Solubility
• Used to assess concentrations in water
• Can be calculated using :
Log S = 0.342 – 1.0374 logKow – 0.0108 (Tm –25) + Σhj
(4.13)
Log S = 0.796 –0.854 logKow – 0.00728 (MW) + Σhj
(4.14)
Log S = 0.693 – 0.96 los Kow – 0.0092 (Tm –25) – 0.00314 (MW) + Σhj
(4.15)
Where :
S : water solubility (mol/L).
Kow : octanol-water partition coefficient.
Tm : melting point (ªC).
MW :s the molecular weight of the substance.
hj is the correction factor for each functional group j.
Example : Water Solubility Estimation
Estimate the Water Solubility for diethyl ether.
Solving :
Equation 4.9 gives the log Kow ≈ 1.05
Using equation 4.14 we can calculate the S :
Log S = 0.796 –0.854 logKow – 0.00728 (MW) + Σhj
Log S = 0.796 – 0.854(1.05) – 0.00728(74.12) + 0.0
Log S = -0.6403
Therfore : S = 0.2289 mol/L. = 16.966 g/L = 16,966.068 mg/ L
Henry’s Law Constant
• Describes the affinity for air over water.
• Can be determined using :
-log H = log (air-water partition coeff) = Σ nihi + Σ njcj
Where :
H : dimensionless Henry’s Law Constant.
ni : number of bonds of type i in the compound.
hi : bond contribution to the air-water partition
coefficient.
nj : number of groups of type j in the molecule.
cj : correction factor for each group.
(4.19)
Example : Henry’s Law Constant Estimation
Estimate the Henry’s Law Constant for diethyl ether.
HH
HH
H-C-C-O-C-C-H
HH
HH
Expressed as a collection of bonds, diethyl ether consists of 10
C-H, 2 C-C bonds, and 2 C-O bonds. The uncorrected value of
log (air to water partition constant) is given by :
-log H = log (air-water partition coefficient) =
10(-0.1197) + 2(0.1163) + 2(1.0855) = 1.2066
log H-1 = 1.2066
Soil Sorption Coefficient
• Used to describe the Soil-Water Partitioning.
• Can be estimated by :
log Koc = 0.544 (log Kow) +1.377
log Koc = -0.55 (log S) + 3.64
log Koc = 0.53 1χ + 0.62 + Σ njPj
(4.16)
(4.17)
(4.18)
Where :
Koc : Soil Sorption Coefficient (μg/g of organic carbon (to μg/mL of
liquid)).
Kow : Octanol-Water Partition Coefficient.
S : Water Solubility.
1χ : first order Molecular Connectivity Index (from literature-appendix ).
nj : number of groups of type j in the compound.
Pj : correction factor for each group j.
Molecular Connectivity Index Calculations
The first step in calculating 1χ is to draw the bond structure of the
molecule. For example, isopentane would be drawn as:
CH3
H3C-CH-CH2-CH3
The second step is to count the number of carbon atoms to which
each carbon is attached. Each C-C bond is given a value of 1 and δi,
is the parameter that defines the quantity of carbon atoms connected to
a carbon atom i. The diagram below gives the δi, values for the
different carbon atoms.
(1)
CH3
H3C-CH-CH2-CH3
(1) (3) (2) (1)
Molecular Connectivity Index Calculations (continued)
The third step is to identify the “connectedness” of the carbons
connected by the bond (δi , δj). For isopentane, these pairs are:
(1,3)
CH3
(2,1)
H3C-CH-CH2-CH3
(1,3) (3,2)
The value of 1χ can then be calculated using the equation :
1χ
= Σ(δi* δj)-0.5
(4.19)
For isopentane,
1χ
= (1/√3) + (1/√3) + (1/√6) + (1/√2) = 2.68
Example : Soil Sorption Coefficient
Estimation
Estimate the Soil Sorption Coefficient for diethyl ether.
Solution :
The molecular structure for diethyl ether is :
CH3-CH2-O-CH2-CH3
Using previously calculated values for log Kow (estimated at 1.0492)
and log S (estimated at -0.6384) we can estimate the soil sorption
coefficients using equations 4.16 and 4.17 :
log Koc = 0.544 (log Kow) + 1.377 = 1.9482
log Koc = -0.55 (log S) + 3.64 = 3.99
Example : Soil Sorption Coefficient Estimation
Using the molecular connectivity we can also estimate the soil
sorption coefficient :
First the molecular connectivity index is calculated using eq. 4.19 :
CH3-CH2-O-CH2-CH3
(molecular structure)
2(C-C), 2(C-O), 2(1, 2) , 2(2, 2)
(connection pairs)
1χ = 2(1/√2) + 2(1/√4) = 2.414
therefore :
Using equation 4.18 to calculate the soil sorption coefficient :
log Koc = 0.53 1χ + 0.62 + Σ njPj
log Koc = 0.53 1χ + 0.62 + Σ njPj = 0.53(2.414) + 0.62 + (-1.264)
log Koc = 0.63542
therefore : Koc = 4.32
Where to look up this
information...
http://www.chem.duke.edu/~chemlib/properties.html
http://www.library.vanderbilt.edu/science/property.htm
http://www.library.yale.edu/science/help/chemphys.html
What do the different Properties mean?
Adapted from the Green Engineering Textbook
Estimating Environmental
Persistence and Ecosystem Risks
To be discussed :
– Atmospheric Lifetimes
– Aquatic Lifetimes
– Overall Biodegradability
– Ecosystems
Estimating Atmospheric Lifetimes
• One way to estimate the atmospheric lifetime of a
compound is to analyze the rate of oxidation of
the substance, specifically the hydroxyl radical
reaction rate.
• Group contributions is again one of the
approaches that can be taken to estimate this
property.
• Using examples, we will show how to estimate
reaction rates and half lives while using the
appropriate correction factors.
Example : Atmospheric Lifetime
Estimation
Dimethylsulfide (DMS, CH3SCH3) produced by phytoplankton degredation
is thought to be the major source of the sulfate and methanesulfonate
aerosol found in the marine boundary layer.
The primary objective of this research effort is to determine the detailed
mechanism of, and final product yields from, the OH initiated gas phase
oxidation of DMS.
At the low NOx levels that are characteristic of the remote marine
boundary layer, reaction with OH is the initial step in DMS oxidation.
OH + CH3SCH3 ⇒ Products
(1)
The OH initiated oxidation of DMS proceeds via a complex, two channel,
mechanism involving abstraction (1a) and reversible addition (1b, -1b). This
can be described by the reaction sequence:
CH3SCH3 + OH ⇒ CH3SCH2 + H2O
CH3SCH3 + OH + M ⇔ CH3S(OH)CH3 + M
CH3S(OH)CH3 + O2 ⇒ Products
(1a)
(1b, -1b)
(3)
Because of this complex mechanism the effective rate coefficients for reaction
(1) and its deuterated analog, reaction (2) depend on the partial pressure of O2
at any total pressure.
OH + CD3SCD3 ⇒ Products
(2)
The two channel reaction mechanism implies that in the absence of O2 we
measure k1a, the abstraction rate. As we add O2 the effective rate increases until
we measure a limiting rate (k1a + k1b).
Estimating Aquatic Lifetimes
• One way to estimate the aquatic lifetime of a compound is
to analyze the rate of hydrolysis of the substance.
• The rate of hydrolysis can be estimated by :
log (hydrolysis rate) = log (hydrolysis rate of a reference compound)
+ Constant * σ
Therefore log (hydrolysis rate) = A + Bσ
(4.20)
Where :
A is rxn and compound class specific(depends on the reference rxn chosen)
B is rxn and compound class specific (depends on type of rxn considered)
σ is a structural parameter commonly used in linear free energy relationship.
Estimating Overall Biodegradability
• It is difficult to do an overall biodegradability analysis.
• It can be estimated using :
I = 3.199 + a1f1 + a2f2 + a3f3 +... + anfn + amMW
(4.21)
Where :
an is the contribution of the functional group (see table ).
fn is the number of different functional group.
MW is the molecular weight.
I is an indicator of aerobic biodegradation rate.
• Different Values (of I) represent different life times :
I value
5
4
3
2
1
Expected degradation rate
Hours
Days
Weeks
Months
Years
Example : Overall Biodegradability
Estimation
Estimate the Biodegradation Index for diethyl ether.
Solution :
Molecular weight of diethyl ether :
MW = 74.12 g/mol
Using equation 4.21, the index can be calculated :
I = 3.199 + a1f1 + a2f2 + a3f3 + ... + anfn + amMW
I = 3.199 + (- 0.0087) - 0.00221(74.12) = 3.0267
Therefor a lifetime of WEEKS
Estimating Ecosystem Risks
Compare the Fish, Guppy and Daphnids mortalities for an acrylate
with log Kow = 1.22 (e.g. ethyl acrylate).
Guppies
log (1/LC50) = 0.871 log Kow – 4.87
log (1/LC50) = 0.871(1.22) – 4.87 = -3.80738
LC50 = 6417.74 µmol/L.
(4.22)
Daphnids
log LC50 = 0.00886 – 0.51136 log Kow
log LC50 = 0.00886 – 0.51136(1.22) = -0.6149992
LC50 = 0.242 millimoles/L = 242 µmol/L.
(4.23)
Estimating Ecosystem Risks Continued
Fish
log LC50 = -1.46 – 0.18 log Kow
log LC50 = -1.46 – 0.18(1.22) = -1.6796
LC50 = 0.021 millimoles/L = 21 µmol/L.
The concentrations yielding 50% mortality are:
Guppies (14 day): 6417.74 µmol/L.
Daphnids (48 hour): 0.242 millimoles/L = 242 µmol/L.
Fish (96 hour):
0.021 millimoles/L = 21 µmol/L.
(4.24)
Environmental Fate and Exposures
Example : If chemicals are released into a river
upstream of a water treament plant, what factors
need to be taken into account to estimate the
potential danger to the community. What
fraction of the chemicals are:
- Absorbed by river sediments.
- Taken up by living organisms.
- Reacted with other compounds.
- Volatilized into the air.
- Biodegraded.
- Removed in the
treatment process.
Classification of Substances
Based on Risk
By examining the table XX, we can use the calculated
properties to qualitatively quantify the risk associated with
the different substances
Three main criteria are normally considered in the
classification of the substances : persistence,
bioaccumultion and toxicity.
There do not exist a given set of regulations or guidelines on
quantifying risk, but the above parameters are used in the
process.
Available Ressources
EPA (persistent, bioaccumulating and toxic substances) :
http://www.epa.gov/pbt/aboutpbt.htm
http://www.epa.gov/opptintr/pbt/
Pollution Prevention, Waste Minimization and
PBT Chemical Reduction :
http://yosemite.epa.gov/R10/OWCM.NSF/0d511e619f047e0d882565
00005bec99/6ad9c10eb8a06bc288256506007def78?opendocument
Environment canada (existing substances evaluation) :
http://www.ec.gc.ca/substances/ese/eng/psap/psap_2.cfm