PPT #2
advertisement

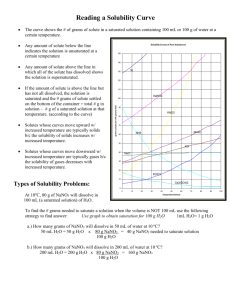
Solubility Rules & Reference Tables Components of a Solution Solute: substance being dissolved Ex: Salt, Sugar Solvent: substance doing the dissolving Ex: Water, Hexane Solubility: How much solute can dissolve under certain conditions of temp. and pressure. Factors Affecting Solubility Surface Area More solute/solvent contact means faster dissolving Crush substance into fine powder Use mortar and pestle Stirring or Agitation: More solute/solvent contact (solids/liquids) However, stirring disturbs dissolved gases and they come out of solution. Temperature of Solvent Higher temperatures will allow more solid solutes to dissolve Gases dissolve better when solvent temperature is colder. Ex: CO2 gas in hot soda (flat) vs. cold soda (fizzy) Pressure Effects gas solubility only Why? Increasing pressure on a gas above a liquid causes more gas molecules to be “pushed” into solution. Ex: CO2(aq) in soda Nature of Solute and Solvent Polar solutes dissolve in polar solvents Nonpolar solutes dissolve in nonpolar solvents Most ionics (but not all) dissolve in polar solvents (molecule-ion attractions) Amount of Solute already Dissolved As particles dissolve in solution fewer solvent molecules are available to dissolve new solute. Miscible: 2 liquids that dissolve (ex: alcohol and water) Immiscible: 2 liquids that do not dissolve (ex: oil and water) Electrolytes: Conduct electricity when dissolved in water Why do they Conduct? Create mobile ions in solution. The more concentrated the solution the more it conducts Includes: Soluble Ionic Compounds (ex: NaCl) Acids (ex: HCl) Bases (ex: NaOH) Who Will Conduct? Which of the following compounds will conduct in solution? (ionic salt, acid, base?) See Ref Tables for common acids/bases C6H12O6 LiBr KOH CH4 H2SO4 NO2 C6H12O6 LiBr KOH CH4 H2SO4 NO2 Will Not (Covalent) Will (Ionic) Will (Base) Will Not (Covalent) Will (Acid) Will Not (Covalent) Using Reference Table G Shows solubility in grams of solute per 100 grams of water at different temps Saturated Solutions: hold max solute possible at that temp. Table G: Solubility curve lines show saturation levels at different temps Saturated Solutions are at EQUILIBRIUM. Rate of dissolving = Rate of crystallization Ex: How many grams of NaNO3 are needed to create a sat. solution in 100g of water at 50 °C? Go to 50 °C and up to NaNO3 and over. Answer: 116 grams Look at The Water!! Table G is for 100 grams of water. Amount of water in your problem may be different and you need to adjust your answer. How many grams of NaNO3 are needed to create a sat. solution in 300g of water at 50 °C? Answer: 116 grams x 3 (three times as much water!) Or you can use a proportion: 116 grams 100 g H20 = x grams 300g H20 Unsaturated Solutions could still hold more solute at that temp. Would fall “below the line” on Table G Ex: 40 g of NaNO3 in 100g water at 50° Supersaturated Solutions hold more solute than they should at that temp. Would fall “above the line” on Table G Ex: 140 g of NaNO3 in 100g water at 50° How do Supersaturated Solutions Form? Create a saturated solution at a high temp. and slowly let solution cool. Certain solutes can stay in solution. Ex: sodium acetate Supersaturated solutions are unstable. Add just one more “seed crystal”, all excess solute will precipitate leaving a saturated solution behind Supersaturated Sodium Acetate solution after seed crystal added Describe These Solutions Saturated, Unsaturated or Supersaturated? 100 g NH4Cl at 70° in 100 g water Falls above the line (Supersaturated) 10g SO2 at 10° in 100g water Falls below the line (Unsaturated) 40g NaCl at 90° in 100g water Falls on the line (Saturated) Concentrated Solutions: have a lot of solute dissolved in the solvent Ex: Saturated solution of KI at 10° 135 grams in 100 g water = pretty concentrated Dilute Solutions: only have a little solute dissolved. Ex: Sat. solution of SO2 at 50° 4 grams in 100 g water = relatively dilute If Temp. Changes How much will precipitate out of solution if a saturated NaNO3 solution at 60° is cooled to 20° ? Reference Table F Describes which ionic compounds are soluble or insoluble in water. Certain combinations of ions hold together so strongly that water cannot dissolve them into solution (insoluble) Is this soluble or not? CaCO3 Carbonate (CO3-2) is insoluble and Ca+2 as a partner is not an exception Is this soluble or not? NaNO3 Nitrate (NO3-1) is always soluble, there are not exceptions Is this soluble or not? Li3PO4 Phosphate (PO4-3) is insoluble, however, Li+1 is a Group 1 ion so it is an exception and the compound is soluble. Soluble or Not? Look out for exceptions! CaSO4 MgSO4 PbCrO4 Li2S NH4OH Insoluble Soluble Insoluble Soluble Soluble CaSO4 MgSO4 PbCrO4 Li2S NH4OH Precipitates Precipitates are insoluble ionic compounds formed in double replacement reactions. Determine which product is the insoluble precipitate by using Table F. When a precipitate forms, you create a heterogeneous mixture. You can separate a precipitate by filtration. The solid will stay on the paper.