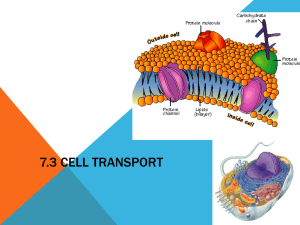
Cell Membrane Osmosis Power Point
advertisement

Osmosis The passive transport of water across a selectively permeable membrane is called osmosis. In order to fully understand this concept, you must know the difference between solute and solvent and the difference between “free” and “bound” water molecules. When salt is added to water, it readily dissolves forming a solution of saltwater. salt Any substance that dissolves in another is called a solute. Any substance that does the dissolving is the solvent. In this case, salt is the solute and water is the solvent. water salt Table salt is known chemically as sodium chloride. Let’s look at what makes up a crystal of table salt. A crystal of salt is composed of many sodium and chlorine ions. Ions are atoms that have a charge. The sodium ions have a positive (+) charge and the chlorine ions have a negative (–) charge. Sodium Chlorine Na Cl Na Cl Na Cl Cl Na Cl Na Cl Na Na Cl Na Cl Na Cl Cl Na Cl Na Cl Na Let’s look at what happens to a crystal of salt as it dissolves in water. salt crystal When a crystal of salt enters water, the (-) ions are attracted to the positive poles of water molecules. water molecules Let’s look at what happens to a crystal of salt as it dissolves in water. When a crystal of salt enters water, the (-) ions are attracted to the positive poles of water molecules. The (-) ion will then dissociate itself from the salt crystal and become surrounded by other water molecules. salt crystal Conversely, the (+) ions are attracted to the negative poles of water molecules. salt crystal Conversely, the (+) ions are attracted to the negative poles of water molecules. The (+) ion will also dissociate and become surrounded by water molecules. salt crystal Conversely, the (+) ions are attracted to the negative poles of water molecules. salt crystal The (+) ion will also dissociate and become surrounded by water molecules. As long as there is sufficient water, this process will continue until all of the ions are dissolved. Dissolved ions When water molecules are clumped around ions, they are considered “bound.” When water molecules are not bound, they are called “free.” Bound water molecules Ions Free water molecules Bound water molecules cannot pass through a selectively permeable membrane. Free water molecules however are permeable. Selectively permeable membrane In osmosis, water flows from the side of the membrane that has the greatest number of free water molecules to the side with the fewest number. Net flow stops when the free water concentration in both areas is equal. Selectively permeable membrane Equalibrium Which side of the membrane shown has the greatest number of free water molecules? Answer: The right side has 10 free water molecules. The left side only has 4 free water molecules. Selectively permeable membrane In which direction will the free water molecules flow through the membrane? Answer: Flow will go from the right side (the most concentrated in free water) to the left side (the least concentrated). Selectively permeable membrane When will net flow stop? Answer: When equilibrium is reached (7 free water molecules on each side). Selectively permeable membrane Will free water molecules continue to flow back and forth across the membrane? Answer: Yes, but at equal rates. Selectively permeable membrane Salt In this example of osmosis, an equal amount of water and salt are placed in a Utube apparatus. In the middle of the Utube are two barriers which prevent the two salt solutions from mixing. In addition, there is a membrane that only allows the flow of free water molecules. Barriers Water Salt Water Membrane What do you hypothesize will happen to the fluid level on each side of the membrane when the barriers are removed? Answer The fluid levels on both sides stay the same. This is because both volumes of water have an equal concentration of free water molecules, so no net movement is observed. Salt Water Membrane This time we will double the amount of salt on the right side of the U-tube. The diluted water on the left side is said to be hypotonic (have less dissolved solutes) compared to the concentrated water on the right. The concentrated water on the right is said to be hypertonic (have more dissolved solutes) compared to the diluted water on the left. Dilute Saltwater hypotonic Concentrated Saltwater Membrane hypertonic Now hypothesize what will happen to the water levels on each side of the membrane when the barriers are removed. Hint: Adding salt results in an increase of bound water molecules and a decrease in free water molecules. Dilute Saltwater hypotonic Concentrated Saltwater Membrane Free Bound hypertonic Answer: The left side has a lower concentration of salt but a higher concentration of free water molecules. Thus the water will flow through the membrane from left to right. Dilute Saltwater Concentrated Saltwater Membrane Let’s run the same experiment again but this time place a pressure gage on the right side. The pressure gage will give an indication of the amount of pressure the water on the left side exerts. We’ll now remove the barriers. Now watch the red gage needle… Dilute Saltwater Concentrated Saltwater Membrane The red needle indicated an increase in water pressure. Notice the water level did not change. This is because the gage prevents the water from moving. Dilute Saltwater Concentrated Saltwater Membrane The blinking red arrow does not represent water flow, but the direction of water pressure instead. Pressure as a result of osmosis is called osmotic pressure. Dilute Saltwater Concentrated Saltwater Membrane Now let’s see what effect osmosis has on living cells. In this beaker of pond water is a microscopic one-celled organism called a paramecium. Paramecia are so small that you need a microscope to see them. Pond water Notice how the two contractile vacuoles inside the paramecium expand and contract. Make a hypothesis as to why they are doing this. Answer: Water constantly enters the paramecium by osmosis. The contractile vacuoles expel the water from the cell. Pond water What will happen to the paramecium if the contractile vacuoles fail to function? Answer: The paramecium will probably expand to the point that it lysis (breaks open). Pond water Let’s add some salt to the pond water. salt The salty pond water is now hypertonic compared to the inside of the paramecium. Will water tend to flow out of the paramecium or into it? Salty Pond water Answer: Water will tend to flow out of the paramecium. H 2O H 2O How will the added salt affect the rate at which the paramecium contracts its vacuoles? Answer: To conserve water, the paramecium will slow the rate at which its vacuoles contract. Before salt was added Compare the rates Salty Pond water After salt was added Assume we now place the paramecium in distilled water. Distilled water is pure water. It does not have any impurities or salts dissolved in it. Distilled water has nothing but free water molecules. Distilled water Salty Pond water Now with the paramecium in distilled water, hypothesize how the rate of vacuole contractions will change. Answer: Too much water is entering into the paramecium. The vacuoles must contract faster to expel the water. In pond water Compare the rates Distilled water In distilled water Osmoregulation is the ability of an organism, like this paramecium, to regulate its internal environment via osmosis. Cheek cells are placed in three different solutions below. Based on what happens to the cells, determine if the solution is hypotonic, hypertonic or isotonic to the cell’s internal environment. Refer to the definitions below. H 2O H 2O H 2O H 2O Isotonic: Having a solute concentration equal to that of another solution. Hypertonic: Having a higher concentration of solute than another solution. Hypotonic: Having a lower concentration of solute than another solution. Answer: Hypertonic Isontonic Hypotonic Unlike paramecia, cheek cells lack contractile vacuoles to regulate osmosis. This makes cheek cells more susceptible to death by shriveling in hypertonic solutions and swelling in hypotonic solutions. Sea water is hypertonic to fresh water. Ocean fish like tuna cannot survive in fresh water. Sea water Fresh water Fresh water is hypotonic to sea water. Fresh water fish like brown trout cannot survive in sea water. The cell below represents a typical plant cell with the following components: Nucleus Cell wall Chloroplast Central vacuole (acqueous) Mitochondria Golgi body Cytosol H 2O H2 O Water moves into the cell… and out of the cell by via osmosis. Let’s place plant cells in isotonic, hypertonic and hypotonic solutions. Isotonic H 2O Hypertonic H 2O H 2O H 2O In an isotonic solution, water moves in and out at equal rates. Hypotonic In an hypertonic solution, water moves out of the cell. In an hypotonic solution, water moves into the cell. In a hypertonic solution, plant cells are plasmolyzed, which is a condition where the plasma membrane pulls away from the cell wall. Isotonic In a hypotonic solution, plant cells become turgid, or swollen. The osmotic pressure that causes this is called turgor. Hypertonic Hypotonic Plasmolyzed cell Turgid cell Match each plant’s state below to the plant cell’s condition in each solution. Answer: Isotonic A Hypertonic Hypotonic Plasmolyzed cell Turgid cell B C Isotonic B Hypertonic Hypotonic Plasmolyzed cell Turgid cell C A Isotonic Isotonic solutions make plants slightly wilted (flaccid). B Hypertonic Hypotonic Plasmolyzed cell Turgid cell Hypertonic solutions make plants very flaccid. C Hypotonic solutions help keep plants firm and upright. A