Characterization of weak acid hydrolyzate fermentation
advertisement

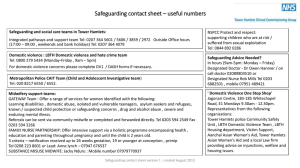
Pretreatment of different materials: An international perspective Mohammad J. Taherzadeh School of Engineering University of Borås School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery A variety of lignocelluloses are attractive in different reagions! School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Complex plants cell walls School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery A challenge by lignin and hemicellulose! Hemicellulose Lignin Cellulose chains School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Challenge of crystallinity! School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Alternative patterns of aggregation Helical form of a 6 by 6 nm nanofibrils is compared with that of nine 2 by 2 helical nanofibrils packed as close as possible, with the same period. A. The fibrils were twisted individually and then the assembly also twisted. B. The individual fibrils were twisted of 90°over 300 nm and then packed as closely as possible C. The fibrils were collectively subjected to twist. School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Alternative patterns of aggregation Cellulose is deposited alone The most efficient load-bearing structure Would not be mechanically stable School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Changes during Isolation • Two stages in isolation will influence the final pattern of aggregation: 1. Elevation of temperature 2. Effect of drying School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Effects of elevated temperature Cellulose is hydrated in its native state at the level of elementary nanofibril Temperature elevation changes the state of aggregation of native cellulose. School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Drying? Drying results in distortion of nanofibrils by: Removing too much of the water needed to lubricate the motion of the nanofibrils relative to each other. School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Changes during Isolation Linear parallel segments, which are artifacts of isolation processes are easily mistaken for naturally accuring crystalline domains. School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Pretreatment in order to: Release cellulose from the structure: Remove or hydrolyze hemicelluloses Remove lignin Reduce cellulose crystallinity Provide enough accessible surface area to absorb the enzymes Adsorption/desorption rates of the enzymes Remove inhibitory compounds following the substrate School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Pretreatment methods (various efficiency on different factors) Physical methods Chemical & Physicochemicals Biological milling: - Ball milling - Two-roll milling - Hammer milling - Colloid milling - Vibro energy milling irradiation: - Gamma-ray irradiation - Electron-beam irradiation - Microwave irradiation Others: - Hydrothermal - High pressure steaming - Expansion - Extrusion - Pyrolysis Explosion: - Steam, Ammonia, CO2, SO2 , Acids Alkali: - NaOH, NH3, (NH4)2SO3 Acid: - Sulfuric, Hydrochloric & Phosphoric acids Gas: - ClO2, NO2, SO2 Oxidizing agents: - Hydrogen peroxide - Wet oxidation - Ozone Solvent extraction of lignin: - Ethanol-water extraction - Benzene-water extraction - Ethylene glycol extraction - Butanol-water extraction - Swelling agents Organosolvs/ Ionic liquids Fungi and actinomycetes (lignin peroxidase, manganese peroxidase, laccase…) School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Cellulose solvents effective for crystallinity Component type Transition metal complexes with amines or NH3 Transition metal complexes with tartaric acid Ammonium hydroxides Alkali hydroxides Acids School of Engineering Sovent Active component Cadoxen Cdtren Cooxen Cupren Cuam Cuen Nioxam Nioxen Nitren Pden Zincoxen [Cd(H2 N-(CH2)2 -NH2)3 ](OH)2 [Cd(NH2 CH2 CH2)3 N](OH)2 [Co(H2 N-(CH2)2 -NH2)2 ](OH)2 [Cu(H2 N-(CH2)3 -NH2)2 ](OH)2 [Cu(NH3)4 ](OH)2 [Cu(H2 N-(CH2)2 -NH2)2 ](OH)2 [Ni(NH3)6 ](OH)2 [Ni(H2 N-(CH2)2 -NH2)3 ](OH)2 [Ni(NH2 CH2 CH2)3 N](OH)2 [Pd(H2 N-(CH2)2 -NH2 ](OH)2 [Zn(H2 N-(CH2)2 -NH2)2 ](OH)2 FeTNa Triton B TEOH Triton F GuOH Na6 [Fe(C4H3O6)3] Trimethylbenzyl ammonium hydroxide Tetraethylammonium hydroxide Dimethyldibenzyl ammonium hydroxide Guanidinium hydroxide NaOH LiOH H3PO4 Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Possible non-aqueuous cellulose solvents Component type Sovent name Unicomponent N-Alkylpyridinium halogenides Oxides of tertiary amines Bi-component Tri-component Active component N-Ethylpyridinium chloride N-Methylmorpholine-N-oxide Triethylamine-N-oxide N-Methylpiperidine-N-oxide Dimethyl sulfoxide (DMSO) containing solvents DMSO/methylamine DMSO/KSCN DMSO/CaCl2 DMSO/TBAF Liquid ammonia/sodium or ammonium salts NH3 /NaI (NH4 I) NH3 /NaSCN (NH4 SCN) Dipolar aprotic solvents/LiCl N,N-Dimethylacetamide/LiCl N-Methylpyrrolidone/LiCl Pyridine or quinoline containing systems Pyridine/resorcinol Quinoline/Ca(SCN)2 NH3 or amine/salt/polar solvent NH3 /NaCl/DMSO Ethylenediamine/NaI/N,NDimethylformamide NH3 or amine/SO2 or SOCl2 /polar solvent Diethylamine/SO2 /DMSO School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Low-temperature Ionic Liquids (80-130 C) 1-Ethyl-3-methylimidazolium acetate [EMIM]OAc 1-Ethyl-3-methylimidazolium chloride [EMIM]Cl 1-Allyl-3-methylimidazolium chloride [AMIM]Cl 1-Butyl-3-methylimidazolium chloride [BMIM]Cl 1-Butyl-3-methylimidazolium bromide [BMIM]Br 1-Butyl-3-methylimidazolium acetate [BMIM]OAc 1-Butyl-3-methylimidazolium tetrafluoroborate [BMIM]BF4 1-Butyl-3-methylimidazolium hexafluorophophate [BMIM]PF6 1-Butyl-3-methylimidazolium methylsulfate [BMIM]MeSO4 Imidazole salts 1-Octyl-3-methylimidazolium chloride [OMIM]Cl School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Milling: Usually part of the process! Milling: Ball milling Two-roll milling Hammer milling Colloid milling Vibro energy milling Functions: Size reduction Degree of crystallinity High energy costs! School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Irradiations Irradiation: – Gamma-ray irradiation – Electron-beam irradiation – Microwave irradiation Usually good results Expensive! Ultrasound might have a good a chance for commercialization (already in the market) School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Hydrothermal process Cooking in liquid hot water generally at 150-210 °C Water under high pressure can: – – – – Autohydrolysis occur due to releasing some carboxylic acids such as acetic acid, Advantages: – – – – Penetrate into the biomass, Hydrate cellulose, Remove hemicellulose (a major function), Remove part of lignin (but not so effective), No addition of chemicals, No neutralization afterward, No corrosion-resistant materials for reactor, Could be combined with e.g. a delignification process Industrial application such as by Danish Inbicon School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery High-pressure steaming (+explosion) Cooking with steam generally at 150-220 °C & 0.5-20 min If followed by explosive releasing of the pressure = Steam explosion Steam explosion is one of the most popular pretreatments Explosion has important function pH reduces due to autohydrolysis Industrtial applications by e.g. – Cambi (Norway) – Chemtex (Italy) – Greenfield (Canada) School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Dilute-acid processes! Similar methods in pulp industries Similar temperature/Pressure as steam explosion! Improvement by addition of: – – – – Dilute-acid (0.1-1% acids: H2SO4, HCl, etc.) Carboxylic acids (e.g. acetic acid) 1-4% SO2 CO2 Functions: – Open up the polymers – Hydrolysis of hemicellulose Potential commercialization by e.g : – POET (USA) – SEKAB (Sweden) – ABENGOA (USA) School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Alkaline cooking! Treatment with alkaline solutions: – NaOH, – Ca(OH)2 (lime) or – Ammonia (AFEX) Popular in pulp industries (kraft process) To remove lignin effectively (+ sometimes a part of the hemicellulose) To reduce crystallinity of cellulose Generally at about 90-130 C for a few minutes to hours High pH (e.g. 11-12) or alkali concentrations 1-20% High amount of NH3 is needed (e.g. 1:1 kg/kg NH3/biomass) Can also be used with explosion School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Oxygen treatments (wet oxidation)! Wet oxidation = treatment with water and air or O2 at 140-200 C for e.g. 30 min. It is exothermic process (because of oxidations) It is a combination of solubilization and degradation reactions Hydrolytic reactions organic acids The hemicelluloses are extensively cleaved to monomeric sugars; The lignins undergo both cleavage and oxidation; Cellulose is partly degraded. School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Organosolves/Ionic liquids! Lignocelluloses are mixed with organic liquid (and water?) and heated to dissolve: – Ligning – And/or cellulose Temperatures of 80-200 °C can be used: – Depends on the solvent used! Simple solvents such as ethanol or acetone can be used! Low Temperature Ionic Liquids are hot research topic today! School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Our latest results for high crystalline cellulose! N-Methylmorpholine-N-oxide (NMO or NMMO) No toxicity Solvent of cellulose Industrial solvent 80-130 C School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Conclusion Lignocelluloses are diffent: Type, Age, Crystallinity Dryness, Type of the cell wall …. Pretreatments have different effectivities on various lignocelluloses, Great developments in pretreatments, but still long way to go… School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery Thank you! Question? School of Engineering Mohammad.Taherzadeh@hb.se Tel: +46-70-7171032 www.hb.se/ih/resourcerecovery