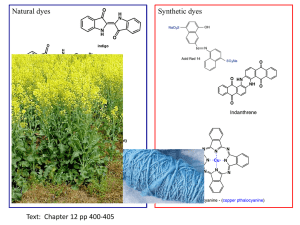
File - TexTile Come
advertisement
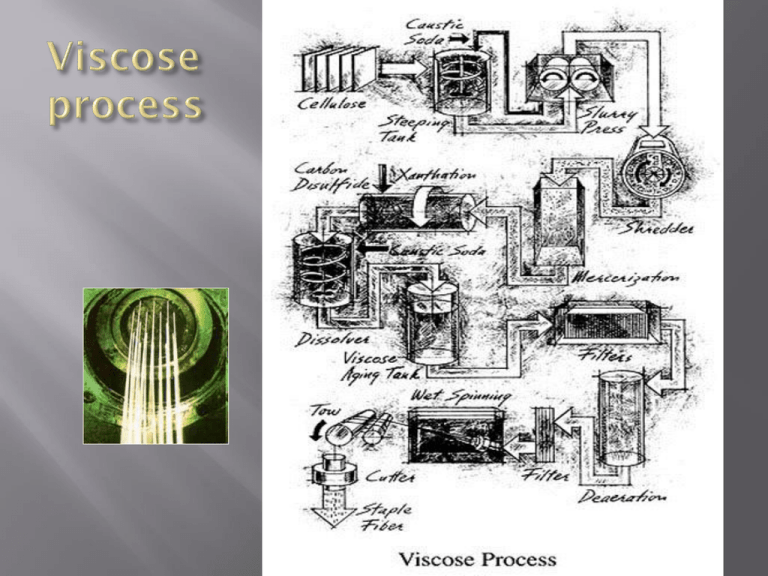
Step 1: Purification of cellulose: • Spruce tress are cut into timber, their barks are removed and then cut into small pieces. • these pieces are treated with solution of calcium bisulphite [Ca (HSO3)2] and cooked with steam under pressure for 14 hrs. The cellulsoe is unaffected by this treatment but the lignin which is present in the wood is converted into its soluble sulphonated compound which is soluble in water and is washed off by purifying the remaining cellulose. • after cooking wood with calcium bisulphite a large amount of water is added, when cellulose pulp is floats, it is sucked through the slots; about 0.2 mm wide. A slurry containing about 30 % cellulose is made which is then treated with bleaching agent and finally converted into paper boards or sheets. Thjs is wood pulp which is normally purchased by manufacturing. Step 2: conditioning of wood pulp The flat, bleached sulphite wood pulp sheets, containing 90 – 94% cellulose are kept in a room at definite temp and humidity for a sufficient time. This is called conditioning . The temperature is maintained by air ventilation at 30 °C. Step 3: Steeping process The conditioned sheets are treated with caustic soda solution (17.5%). This is called steeping process. Uniform impregnation of material with solution is essential . Sheets are allowed to sock until they become dark brown in colour. It takes about 1-14 hours. Now the degree of polymerization of wood cellulose is about 1000. this cellulose contain about 8-10% hemicelluloses which dissolve in caustic soda solution used in steeping process. The excess caustic soda solution and hemicelluloses dissolved in it is removed by pressing sheets in hydraulic press. The sheet are then transformed to a shredding machine for next opertaion. 100 kg of sulphite pulp gives about 310 kg of soda cellulose containg some caustic soda liquor. Step 4: Shredding process The soda cellulose falls into shredding machine which contain two arms, bearing toothed wings which cut the pulp sheet into small bits. Thus they convert into fluffy snow like masses of alkali cellulose. In 2-3 hours the sheets are broken into fine crumbs. Step 5: Ageing process During this process the DP is decreased from 1000 to about 300 by the oxygen present in the air, contained in the drum. The soda cellulose is kept in steel drums at 23°C for 48 hrs. Step 6: churning process This process is also called xanthation or sulphidising process. After ageing the crumbs are transferred to rotating, air-tight hexagonal churners which are similar to cement mixtures. Carbon disulphide (10% of the weight of the crumbs) is added to the mixture and churn together for 3 hrs by rotating the mixture at slow speed of 2 rev/min. sodium cellulose xanthate is formed during this process and colour of the product changes from white to light yellow, to deep yellow and then to reddish orange. After churning is over, the ill-smelling vapors of carbon disulphide is removed by applying vacuum. Step 7: Mixing or dissolving process The orange product from churner is in the form of small balls. They fall into vessel, a dil solution of caustic soda is also added and the contents are stirred for 4-5 hrs. the sodium cellulose xenthate dissolve to give a clear brown thick viscose liquor called viscose. It contains about 6.5% caustic soda, 7.5 % cellulose. Viscose from eight different vissolvers are mixed together in one big mixer and undissolve fibres are removed through filtration. Step 8: ripening process The viscose solution require to ripen to give the best solution for spinning. Ripening is carried out by storing the viscose solution for 2-5 days at 10 to 18°C. During this period the viscosity drops and then rise back to the original value due to sedimentation. The viscose solution is again filtered and now its ready for spinning. Step 8: spinning process In spinning viscose solution is forced through the spinneret by compressed air (40 – 75 lb/in pressure). The spinneret have many fine holes (0.05 -0.1 mm) and it submerge into a solution containing the following chemicals; 10% sulphuric acid 18% sodium sulphate 1% Zinc sulphate 2% glucose 69% water Temperature is kept at 40-45°C. sodium sulphate precipitates the dissolve d sodium sulphate xanthate and sulphuric acid converts the xanthate into cellulose, carbon disulphide and sodium sulphate. Glucose gives softness and pliability to filaments. While zinc sulphate gives added strength. The remaining process include washing with water to remove chemicals followed by washing with sodium sulphide solution (25-30 gm/liter) at 65°C to free filaments from residual sulphur, the process is called desulphurizing. Macro-structure of viscose Viscose is a fine regular filament or staple fibre (crimped confrigation). The length of staple fibre cut from viscose filaments depends upon the required end-use. Usually they are cut into lengths similar to cotton, flex and wool. Viscose fibre dia ranges form 12 μm to 22 μm, depending upon fibre end use. This gives fibre to breath ration in excess of 2000:1, ensuring even the shorter fibre will spin satisfactorily into yarn. The colour of the filaments tends to be slightly off-white. This is attributed to their translucency which permits some light to pass through the filaments before it is reflected. Viscose filaments have many longitudinal striations (tiny groove) which give them their characteristic appearance. They are responsible for irregular perimeter of their nearly round to oval cross-section. Such skin feels more comfortable than the much more intimate contact of non-striated fibres. The polymer system The polymer system is similar to cotton. However the viscose polymer does not have spiral configuration of the cotton polymer. The viscose polymer is very amorphous, being about 35-40% crystalline and about 60-65% amorphous. Its short polymer chains make its difficult to achieve a more crystalline polymer system. Tenacity: Polymer system of viscose is very amorphous and filament s and fibers are weak. When wet, viscose is only half as strong as when dry. Elastic plastic nature: It is limp handling fiber. Polymer system not sufficiently long so hydrogen bonds formation is restricted due to which viscose fiber has crisper handle. When filament is put under strain, the polymer do not come to original position. so the filaments disarranged and will become stretched, wrinkled and distorted. Hygroscopic nature: The polymer system is very absorbent due to amorphous region and polar polymers. Static electricity property is same as cotton. Chemical properties: With regard to dyeing and printing, the regenerated cellulose fibers will generally color more brightly, even when delutered, than even their mercerized cotton . This is due to greater amount of incident light reflected by viscose, even when delustered.