Biological Molecules - Princeton High School
advertisement

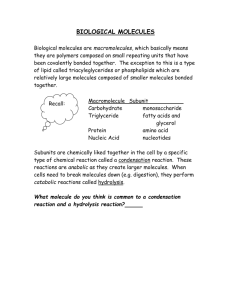
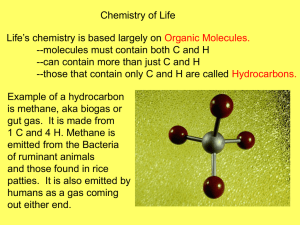
Carbohydrates, Lipids, Proteins, and Nucleic Acids Is it made of carbohydrates? Organic compounds composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio. A carbohydrate with 6 carbon atoms would have… ______ hydrogen atoms and ______ oxygen atoms Source of ENERGY Structural components of cells Monomer of carbohydrate (simple sugar) Most have 6 carbon atoms Most common: 1) Glucose (main source of energy) 2) Fructose (found in fruits) 3) Galactose (found in milk) Glucose, fructose, and galactose have the same chemical formula. C6H12O6 Same formula but different structural forms. Disaccharide: two monosaccharides bond to form a double sugar. Example: sucrose Polysaccharide: complex molecule composed of three or more monosaccharides. Glycogen: animals store glucose in the form of the large, complex molecule – glycogen Starch: plants store glucose molecules in the form of starch. Cellulose: large polysaccharide made by plants, which provide strength and rigidity to plant cells. White bread is a simple carbohydrate (little nutritional value, digested more quickly, converted to fat more quickly) Whole grain bread is a complex carbohydrate (high in fiber, vitamins and minerals, provide more energy, digested slowly) Large organic molecules Nonpolar – do NOT dissolve in water Include phospholipids, triglycerides, waxes, steroids Higher ratio of carbon and hydrogen to oxygen than carbohydrates…. More C-H bonds Store more energy per gram than most other organic compounds Carboxyl End -COOH Hydrocarbon End C-H Polar Nonpolar Hydrophilic Hydrophobic Saturated: Each carbon atom is single-bonded to 4 other atoms; straight chain; molecules are close together; solid at room temperature Unsaturated: Double bonds in the C chain; kinked chain; molecules are farther apart; liquid at room temperature http://www.youtube.com/watch?v=ESPNqKU luRs Let’s list some more differences in the form and function of fatty acids Triglycerides (FATS) 3 fatty acids molecules joined to 1 glycerol Saturated triglycerides are composed of saturated fatty acids (butter, other dairy products, fat from red meat) Unsaturated triglycerides are composed of unsaturated fatty acids (mostly found oils and in plant seeds) Phospholipids Glycerol is connected to 2 fatty acid molecules and a phosphate group. The plasma membrane of the animal and plant cells are composed of a phospholipid bilayer. Waxes Composed of a long fatty acid chain and a long alcohol chain. Waterproof and provide protection. Found on the surface of plants and in the ear canals of many animals (including humans). NOT composed of fatty acids Four fused carbon rings which are attached to other functional groups Cholesterol (found in the cell and plasma membrane) Hormones such as testosterone and estrogen Is it made of Proteins? Organic molecules compose of hydrogen, carbon, oxygen and NITROGEN Monomers = AMINO ACIDS Examples: Hair Horns Skin Muscles ENZYMES (to learn more about later!) 20 different AA Central carbon, C, that is covalently bonded to 4 other groups 1. 2. 3. 4. AA Hydrogen = blue Carboxyl (-COOH) = green Amine (-NH2) = yellow R group = red (varies in each AA and determines the AA’s form and function can also be illustrated as a ball Dipeptide: two amino acids bond Polypeptides: long chains of amino acids (made up of 1 or more dipeptide) Making proteins: what builds them? Reaction: condensation or hydrolysis Water is released or used Some proteins are very large, some are small. Protein shape: Influences its function (form and function) Shape can be influenced by factors like temperature and solvent) … for example, egg white is clear when it is uncooked, and white when it is cooked RNA or protein molecules that act as biological catalysts Catalyst – speeds up the reaction by lowering the activation energy Essential for cellular function Each Enzyme bonds with a specific Substrate for form the Active Site Speed Enzyme bonds to substrate and the enzyme shape changed slightly The chemical bonds in the substrate are weakened Lowers the activation energy What up reactions – how???? happens after the reaction? Enzyme releases the products Enzyme is unchanged… BUT changes in temperature and pH can change the enzyme (denature it) and it may not function properly or at all Large and complex biological molecules Store and transfer important information in the cell Genetic Code Two types – both are polymers 1. DNA - DeoxyriboNucleic Acid 2. RNA - RiboNucleic Acid DNA is transcribed into RNA, which is translated into PROTEINS Each nucleotide is made of: a phosphate group, a five carbon sugar, and a ring-shaped nitrogen base Nitrogen bases A - Adenine C – Cytosine G – Guanine T – Thymine U - uracil