lecture 12 catalysis_transformation of alkenes_alkynes
advertisement

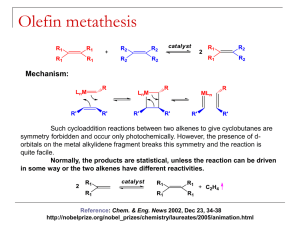
Lecture 12 CATALYSIS 2. TRANSFORMATION OF ALKENES AND ALKYNES Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display. I. METATHESIS OF ALKENES, ALKYNES AND CYCLOALKENES A. Alkene metathesis B. Alkyne metathesis C. ROMP D. Alkyne polymerization II. ALKENE DIMERIZATION AND OLIGOMERIZATION A. Ethylene dimerization B. Oligomerization by successive insertions III. ALKENE ISOMERIZATION A. Double bond migration by -elimination B. Allylic C-H activation C. Cis-trans isomerization via metallocarbenes IV. OLEFIN POLYMERIZATION TRANSFORMATION OF ALKENES AND ALKYNES Metathesis – derived from the greek word meaning “to place differently” or “to transpose” Metal-catalyzed exchange of alkylidene and alkylidyne units in alkenes and alkynes: METATHESIS OF ALKENES AND ALKYNES Depending on the nature of the applied alkene and the reaction conditions, metathesis reactions can give different results, allowing a structuring of the area: ALKENE METATHESIS ALKENE METATHESIS Chauvin Mechanism (1970) A pathway that involves a metallacyclobutane intermediate A metallacyclobutane intermediate was isolated and characterized by Schrock (1989) ALKENES METATHESIS A metal alkylidene unit M=CH2 functions as the catalytically active center Mo, W, Re, Rh have proven to be particularly useful central metal atoms ALKENES METATHESIS Early metathesis catalysts were derived from transition metal halides and carbanion donors (WCl6 / Et2Al / EtOH) ALKENE METATHESIS ALKENE METATHESIS - CATALYSTS Both complexes have low coordination number (CN = 4) allows a facile access to the central metal atom. Spectator ligands aids (imido , oxo ) in the formation of the metallacycle intermediate TRANSFORMATION OF ALKENES AND ALKYNES CM has been used extensive the industry in the form of Higher Olefin Process (SHOP) – a combination process consisting of oligomerization, isomerization, and metathesis steps. The metathesis step: CROSS METATHESIS (CM) If a shop process is followed by hydroformylation, fatty alcohols with 8-22 carbon atoms are formed! In the laboratory, CM has a limited application. Products are obtained as mixtures of Z/E isomers CROSS METATHESIS In contrast to CM, Ring Closing Metathesis (RCM) has become a standard method in organic chemistry. RING-CLOSING METATHESIS Newer catalysts has inspired natural products synthesis: RING CLOSING METATHESIS Assymetric ring closure metathesis (ARCM) has also been developed using chiral catalysts: RING CLOSING METATHESIS Ring-opening metathesis is the reverse of RCM. A cross-metathesis with ethylene forms terminal dienes Ring strain favors ring opening and are specially common with nobornenes and cyclobutenes. Synthetic utility is limited by the formation of different CM and self-methathesis products RING OPENING METATHESIS In some cases selectivity are achieved: RING OPENING METATHESIS Self metathesis; use of open chain alkene substrate is avoided. The C=C double bond in the monomer is conserved in the polymer. The catalytically active species is fixed to the end of the growing chain (“living polymer”) As soon as a certain monomer is consumed, a different monomer can be used to make block coplymers. Can be deactivated by reaction with a carbonyl group to yiled M=O (Wittig reaction). RING OPENING METATHESIS POLYMERIZATION ROMP mechanism: RING OPENING METATHESIS POLYMERIZATION ROMP in the industry: The C=C double bond in this norbornene rubber allows for cross-linking TRANSFORMATION OF ALKENES AND ALKYNES ROMP in the industry: TRANSFORMATION OF ALKENES AND ALKYNES Metathesis involving C C triple bonds can proceed symetrically (Yne YneM) and in the mixed form (Ene YneM) Metathesis using dissymetrical alkynes using MoO3 or WO3 as heterogeneous catalysts: ALKYNE METATHESIS Prototype Catalyst: [W(C-tBu)(O-tBu)3] TRANSFORMATION OF ALKENES AND ALKYNES Mechanism: TRANSFORMATION OF ALKENES AND ALKYNES The presence of a double and triple bonds in the reactants presents challenges: selective metathesis TRANSFORMATION OF ALKENES AND ALKYNES Metathesis also applies to nitriles: TRANSFORMATION OF ALKENES AND ALKYNES Mixed (EneYneM): ALKENE – ALKYNE METATHESIS Example:. [Ru(CO)3Cl2l2 catalyzes the skeletal rearrangement of 1,6- and 1,7-enynes to form vinylcycloalkenes (Murai, 1994) : ALKENE – ALKYNE METATHESIS SAMPLE PROBLEM: Assume 2-pentene and 2-hexene undergo metathesis. AT equilibrium what are all the possible alkenes that would be present, neglecting stereochemistry about the double bond? Remember to consider self metathesis reactions. SAMPLE PROBLEM: What is the product of cyclooctene metathesis? Two mechanisms: - via metallacyclopentane intermediate - insertion of 2 alkene molecules into M-H ALKENE DIMERIZATION AND OLIGOMERIZATION ALKENE DIMERIZATION VIA METALLACYCLOPENTANE Involves coordination of two ethylene molecules, oxidative coupling, -elimination then reductive elimination. Metal has a low number of valence electron and at least two non-bonding electrons, necessary for oxidative coupling ALKENE DIMERIZATION AND OLIGOMERIZATION Same as polymerization but limited to insertion of two monomeric olefins into the M-H bond. This system is made catalytic by the elimination step. Key point: -elimination should be faster than 3rd alkene insertion. ALKENE DIMERIZATION BY SUCCESSIVE INSERTION Isomerization can occur via migration of the double bond – terminal olefin to an internal olefin. ALKENE ISOMERIZATION 16e hydride complexes isomerize terminal olefins via reversible insertion of the olefin into the M-H bond followed by elimination. Mixture of cis and trans is obtained with the more stable trans form being major. ALKENE ISOMERIZATION by -ELIMINATION Example: ALKENE ISOMERIZATION by -ELIMINATION Catalyst do not contain a hydride ligand. M should have at least two vacant coordination sites like the 14e species Fe(CO)3 ALKENE ISOMERIZATION by ALLYLIC C-H ACTIVATION CIS TRANS or Z/E ISOMERIZATION via METALLOCARBENES Ziegler and Natta Polymerization catalyst: TiCl3/Et2AlCl – a heterogenous mixture Proposed mechanism: (Cosse, 1975) ZIEGLER – NATTA TYPE ALKENE POLYMERIZATION Watson, 1982, DuPont: soluble in initiator, LuCp*2CH3 ZIEGLER – NATTA TYPE ALKENE POLYMERIZATION ZIEGLER – NATTA TYPE ALKENE POLYMERIZATION ZIEGLER – NATTA TYPE ALKENE POLYMERIZATION ZIEGLER – NATTA TYPE ALKENE POLYMERIZATION ZIEGLER – NATTA TYPE ALKENE POLYMERIZATION ZIEGLER – NATTA TYPE ALKENE POLYMERIZATION ZIEGLER – NATTA TYPE ALKENE POLYMERIZATION ALKENE DIMERIZATION AND OLIGOMERIZATION ALKENE DIMERIZATION AND OLIGOMERIZATION ALKENE DIMERIZATION AND OLIGOMERIZATION ALKENE DIMERIZATION AND OLIGOMERIZATION ALKENE DIMERIZATION AND OLIGOMERIZATION ALKENE DIMERIZATION AND OLIGOMERIZATION ALKENE DIMERIZATION AND OLIGOMERIZATION ALKENE DIMERIZATION AND OLIGOMERIZATION ALKENE DIMERIZATION AND OLIGOMERIZATION ALKENE DIMERIZATION AND OLIGOMERIZATION TRANSFORMATION OF ALKENES AND ALKYNES TRANSFORMATION OF ALKENES AND ALKYNES TRANSFORMATION OF ALKENES AND ALKYNES TRANSFORMATION OF ALKENES AND ALKYNES TRANSFORMATION OF ALKENES AND ALKYNES TRANSFORMATION OF ALKENES AND ALKYNES TRANSFORMATION OF ALKENES AND ALKYNES