Oil Refining + Octane Numbers
advertisement

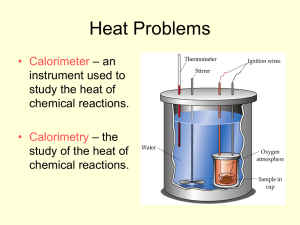
Oil Refining + Octane Numbers Fractional Distillation of Crude Oil • How to become a millionaire .......... Find a crude oil well and know how to fraction it!! • Crude oil found in large quantities in Iran , USA, Nigeria, Russia, North Sea Fossils • Crude oil was formed from the bodies of tiny sea creatures that died millions of years ago • Crude oil is pumped from underneath the ground as is a thick black substance with an unpleasant smell • It does not burn easily and must be undergo fractional distillation to separate out all the useful components • Fractional Distillation • The crude oil is heated in a furnace and starts to evaporate, the longer carbon chains are heavier and do not rise as easily as shorter (lighter) carbon chains • This means that the longer chains carbon fractions are collected first while the shorter chains get collected higher up the column • Remember larger hydrocarbons have higher boiling points while smaller hydrocarbons have lower boiling points Fractionating Column NB. You do not need to memorise the temperatures but you do need to learn the fractions and the uses of the products of each fraction Fractionating Column at Whitegate Oil Refinery Refinery Gas • Top of the column, methane, ethane, propane + butane are gases at 25⁰C • Some used as fuel mostly bottled for sale • Since these are odourless sulfur compounds called Metacarptants are added for safety • Bottled gas is mainly a mix of propane + butane which are liquefied under high pressure Petrol (Light Gasoline) • Used as motor fuel • A mixture of at least 100 compounds mostly hydrocarbons of 5-10 carbons Naphta • Very useful to petrochemical industry • It is a source for a huge number of useful chemicals eg, medicines, plastics, synthetic fibres, detergents, solvents etc. • 7-10 carbons in length Kerosene/Parrafin • Used as aviation fuel and in certain lamps • 10-14 carbon length • NB as the number of carbon atoms increases so does the boiling point as more heat is required to break the larger number of bonds Diesel Oil/GasOil • More difficult to vaporise than petrol • Therefore diesel engine has a different design • Trucks, buses, trains and some cars use diesel • 14-19 carbon length Lubricating Oil • 19-35 carbons in length • Cannot be vaporised easily so cannot be used as a fuel • Used as a lubricant to reduce wear and tear Fuel Oil • 30-40 carbons in length • Used in ships, power stations, heating plants, oil heating in homes Bitumen • More than 35 carbon atoms in length • Very high boiling point • Used in Tar to resurface roads When an oil well is discovered.. • Refinery Chemists analyse crude oil to determine the quality of the oil and assess the quantity of each product in the oil Octane Number • Petrol obtained from the fractionating column is not a very efficient fuel • In the internal combustion engine in a car the power to turn the wheels is produced by the explosive reaction between air and petrol Knocking • Smooth running of an engine depends on this explosion occurring at exactly the right time • If the explosion occurs too early the pistons vibrate and a metallic noise is heard from the engine • Early explosion can occur if the petrol + air explode when they are compressed instead of waiting for the spark • This early explosion is referred to as knocking or auto-ignition • Straight chain alkanes such as nonane, octane and heptane ignite very easily and explode too soon • Branched cahin alkanes such as 2,2,4trimethylpentane (iso-octane) do not tend to auto-ignite • Petrol mixtures with large amounts of branched chain alkanes are more efficient than those which contain straight chain molecules • To indicate the efficiency of a particular type of petrol a number called the Octane Number is assigned to it Octane Number • The octane number of a fuel is a measure of the tendency of the fuel to resist knocking • Since 2,2,4-trimethylpentane is a very efficient fuel it is assigned an octane number of 100 • Heptane is not an efficient fuel and is assigned an octane number of 0 • The octane number of a fuel is calculated by comparing it’s efficiency in an engine to 2,2,4trimethylpentane and heptane What should my petrol be?? • Chemical analysis can also indicate the octane number of a fuel • Good quality petrol should have an octane number of 97 • The shorter the alkane chain the higher the octane number • Which do you think would have a higher octane number hexane or pentane? • The more branched the chain the higher the octane number • Which do you think would be the best fuel heptane, 3-methylhexane, 2,3,-dimethylpentane • Cyclic compounds have a higher octane number than straight chain compounds • Which is a better fuel? Hexane or cyclohexane?? Making Petrol • 1920’s it was found that adding small amounts of lead compounds to petrol helped reduce the amount of knocking • Lead pollution from car exhausts had potential damage to health and was phased out since 2000 • Lead also damaged catalytic converter in car exhausts • Now have unleaded petrol Increasing octane number of fuel • This is now achieved in one of the following 4 ways; 1. Isomerisation 2. Catalytic Cracking 3. Reforming 4. Adding Oxygenates Isomerisation • This involves changing straight chain alkanes into their isomers • Alkanes are heated in the presence of a suitable catalyst and the chains break • The chains are allowed to reconnect but they are more likely to reform in branched-chains than in straight-chains • This is commonly done on a large industrial scale with pentane and hexane (NB fig 21.29) Catalytic Cracking • Catalytic cracking is the breaking down of long chain hydrocarbon molecules into short chain molecules for which there is greater demand • In oil refineries the heavier fractions ( such as fuel oil, diesel oil and kerosene) are heated in the presence of a catalyst • The short chain alkanes produced tend to be highly branched and hence have a high octane number Reforming (Dehydrocyclisation) • Reforming involves the use of catalysts to form ring compounds • Straight chain alkanes are converted to cycloalkanes and these are converted to aromatic compounds • Aromatic compounds have high octane numbers and petrol contains 3-4% benzene, because benzene is a carcinogen this is a cause for concern • Hydrogen may also be produced in reforming reaction, this is a useful substance and may be piped away for various purposes Adding Oxygenates • An oxygenate is any fuel that contains oxygen in its molecules • 3 oxygen containing compounds, methanol, ethanol and MTBE are commonly added to petrol to increase its octane number • Another advantage to adding oxygenates to fuel is that they cause very little pollution when they burn and are cleaner fuels