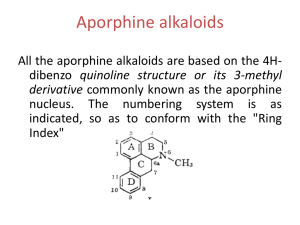
Alkaloids * Natural nitrogenous secondary metabolites from plants
advertisement

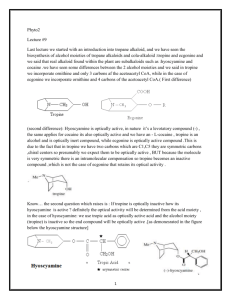
Alkaloids – Natural nitrogenous secondary metabolites from plants and microbes Some important classes of alkaloids Class Piperidine alkaloids Pyrrolidine/tropane alkaloids Precursors L-lysine (C5N) Pyridine alkaloids L-Trp or L-Asp Examples Piperine _____________ L-ornithine (C4N) Cocaine, scopolamine Niacin (Vit. B3), nicotine Catecholamines L-Tyr (C6C2N) and tetrahydroisoquinolines Opiates 2 L-Tyr units Dopamine, adrenaline, mescaline anhalamine Morphine, tubocurarine Phenylalanine-derived L-Phe (C6C3N) Capsaicin, ephedrine Indole alkaloids Quinoline alkaloids Purine alkaloids L-Trp “ L-Gly, L-Gln, L-Asp Serotonin, ergotamine, LSD, vinblastine quinine, camptothecin Caffeine, theobromine Building blocks from the acetate, shikimate, or deoxyxylulose phosphate pathways are also frequently incorporated into the alkaloid structures. Many alkaloids acquire their N via transamination reactions (catalyzed by Vitamin B6). Most alkaloids are quite toxic and produced by the plant as a defense against herbivores. Key biological activity of alkaloids: interaction with CNS • Most of the biological effects of alkaloids are due to their similarity to neurotransmitters in the human body. • They can either mimic or block the effects of neurotransmitters, or cause fluctuations in the normal levels of neurotransmitters. • This leads to numerous physiological and psychological effects Role of neurotransmitters: to transmit nerve impulses across the synapse (space) between neurons in brain, nervous system Structures: mostly small molecules containing amino or ammonium functionalities Action: They are released from nerve endings and bind to receptors on the surface of another neuron in the network Agonists bind to and stimulate receptors Antagonists block receptors Not all alkaloids affect CNS, but many do! Neurotransmitters that have effects on mood, thought processes (a catecholamine) (an indole, aka 5-hydroxytryptamine or 5-HT) Parkinson’s disease – dopamine transmission is inadequate, can be improved by L-DOPA Depression – treatment with SSRIs increases dopamine in the synaptic cleft Neurotransmitters that control physiological effects throughout the body a catecholamine a catecholamine a quarternary ammonium salt Norepinephrine malfunction is implicated in anxiety and mood disorders, bipolar disorder Acetylcholine: low levels may cause memory lapses associated with Alzheimers’, can be improved with cholinesterase inhibitors Alkaloids derived from lysine (piperidine alkaloids – C5N) Formation of the 6-membered ring (also see Lobelia) Formation of piperine: C6C3 unit is lengthened and linked with 2o amine to form amide linkage Piperine: from black pepper (Piper nigrum) Spicy flavor and CYP3A4 inhibitor The pungency of piperine is caused by activation of the heat and acidity sensing TRPV ion channel found on nociceptors (pain sensing nerve cells)[ Has insecticidal properties Produced from three different building blocks Tropane alkaloids (Solanaceae family) Formation of first ring (pyrrolidine ring) • Tropanes are bicyclic non-aromatic alkaloids. They are not common in edible plants, but are found in some botanicals and medicinal herbs. • The most common natural tropane alkaloids are (-)-hyoscyamine and (-)scopolamine (also known as hyoscine). High concentrations of these alkaloids have been found particularly in Datura species. • Hyoscyamine is the major alkaloid in most parts of Datura stramonium (thorn apple or Jimson weed); scopolamine is the major alkaloid in other Datura spp. Assembly of the tropane skeleton found in cocaine, hyoscyamine Chain building by acetyl Co-A units leads to second ring in bicyclic tropanes From leaves of Erythoxylum coca. Well-known CNS stimulant, also anaesthetic Extracts of coca leaves used in original Coca-cola recipe (cola provided the caffeine) but in 1906 the Coca was eliminated. Structural similarity to acetylcholine allows tropanes to block muscarinic ACh receptors, providing anaesthetic effect. Found in belladonna (Atropa belladonna), henbane and Datura species Can be used as sedative or external pain relief, but they are toxic and have psychotropic effects (hallucinations, etc.) Effects of tropane alkaloid contamination in animal feed From European Food Safety Authority website http://www.efsa.europa.eu/EFSA/efsa_locale-1178620753812_1211902036472.htm • “Tropane alkaloids” are a group of > 200 compounds best known for their occurrence in the family Solanaceae, comprising over 100 genera including Datura. • Datura plants are toxic for animals if ingested in large amounts. Their seeds contain significant amounts of hyoscyamine and scopolamine, and can be found as botanical impurities in feed materials, particularly in soybean and linseed products. • Reports on adverse health effects in animals refer mostly to accidental intoxications following the consumption of Datura plants rather than to the contamination of feed. • Overall, pigs have been shown to be among the most sensitive species to Datura poisoning. Effects of tropane alkaloid contamination in animal feed (cont’d) From European Food Safety Authority website http://www.efsa.europa.eu/EFSA/efsa_locale-1178620753812_1211902036472.htm • As competitive antagonists for muscarinic acetylcholine receptors, tropanes prevent binding of acetylcholine, thus affecting the function of smooth muscles and exocrine gland cells, heart rate, respiration and functions in the CNS. • Most common symptoms reported: dryness of the mucosa in the upper GI and respiratory tract, constipation and colic (in horses), pupil dilation, alterations in heart rate, and CNS effects such as restlessness, irritability, ataxia, seizures and respiratory depression. • Tropane alkaloids are readily absorbed following oral ingestion, but have a short biological half-life and are rapidly biotransformed or excreted. • Exposed animals are likely to exhibit symptoms and be removed from the food supply, therefore, it is unlikely that residues of tropane alkaloids in edible tissues, milk and eggs constitute a risk for consumers. More on tropanes...belladonna & henbane Background and Occurrence • Atropa belladonna is known as Deadly nightshade • Related to the tomato (also a member of Solanaceae) • Native to: Europe, North Africa, Naturalized in North America • Morphology: Branching herbaceous perennial, berry bearing, ~1.5m tall • It’s highly toxic! All parts of the plan contain tropane alkaloids atropine and hyoscyamine. Berries are sweet, but toxic • Historical use: Ladies “at court” in Europe applied to the eyes to dilate pupils, “enhance beauty” • Anticholinergic agent: Causes ataxia (loss of coordination) • Also causes pupil dilation, double vision, increased body temperature, confusion. Competes with ACh, reversibly inhibits neuron activity Medicinal Uses of Atropine Antitoxin for cholinesterase Inhibitors ie: physostigmine, neostigmine Organophosphate insecticides Cosmetic application to eyes Gastrointestinal sedative (for antacids) External pain relief (root preparation) Henbane & Jimson weed also contain tropanes Henbane (Hyosycamus niger) contains primarily hyoscyamine, a stereoisomer of atropine, and scopolamine (-)hyoscine. Henbane was thought to induce hallucinations & visions of flight, associated with witches. Could be used to treat GI spasms if it wasn’t so toxic. Jimson weed (Datura) grows wild throughout Europe, Americas. Thieves used it in the Middle Ages to drug & rob people. Some South American tribes used it to calm down unruly kids due to mild sedative effect at low doses. Henbane Cocaine • From Erythroxylum coca • Historically, field workers would consume while picking the leaves • Leaves were extracted with gasoline, then acidified with aqueous acid to purify out the cocaine HCl salt • Salt converted back to “free base” by using CaCO3 • Ether solution of free base evaporated down -> crack Cocaine has several physiological & psychological effects: • Stimulant: increases norepinephrine release • Anesthetic: binds to acetylcholine receptors, decreases Na+ transport • Mood-enhancing/addictive: increases dopamine levels by blocking reuptake Synthetic analogues of cocaine used as local anaesthetics have muscarine structural motif: procaine/novocain Local anaesthetic used to numb skin, rapidly absorbed. Also used as anti-arrhythmia agent after a heart attack Alters signal transduction in nerve membranes by blocking fast voltage-gated sodium channels, stopping pain signals Used mainly as a topical anaesthetic Found in anbesol, cepacol, solarcaine Both are sodium channel blockers, used mainly in dentistry Cocaine and addiction • Addiction from cocaine and similar drugs arises from its effects on dopamine transmission. • Normally, dopamine is released from the transmitting neuron, crosses the synapse and binds to receptors on the receiver. • Excess dopamine is transported back to the transmitting neuron. • Cocaine blocks the dopamine transporters, inhibiting the re-uptake of dopamine by the transmitter. • This results in increased dopamine levels in the synapse euphoria • Reward system results in addiction • Users get used to higher levels of dopamine and “crash” when stimulus is removed. It’s the real thing... • Cola (kola) is the dried cotyledon from seeds of Cola spp. (Sterculiaceae), e.g. C. nitida & C. acuminata, trees cultivated mainly in West Africa and the West Indies. • Fresh cola seeds are chewed in tropical countries as a stimulant • Cola seeds (nuts) contain up to 3% caffeine & 0.1% theobromine, partly bound to tannins. On drying, some polyphenol oxidation occurs, forming a red pigment, and free caffeine is liberated. • Vast quantities of dried seeds are processed for the preparation of cola drinks, e.g. Coca-Cola and Pepsi-Cola. • Coca-Cola was invented by Atlanta pharmacist John Pemberton in 1886. It originally contained cocaine (from E. coca leaf) and caffeine from the kola nut (5 oz. coca leaf/gallon of syrup). It once contained ~ 9 mg cocaine per glass, but in 1903 it was removed. • After 1904, Coca-Cola started using "spent" leaves left over from the cocaine-extraction process. A cocaine-free coca leaf extract is still used for flavoring. Alkaloids from shikimate precursors via transamination Capsaicin • Produced by red hot chili peppers (Capsicum annuum) of Solanaceae • Secondary metabolite, probably produced as a deterrent against herbivores & fungi • Has analgesic properties • Used medicinally in creams to treat neuralgia or neuropathy caused by diabetes, herpes • Also in topical pain-relieving preparations for arthritis. • The initial burning effect of capsaicin affects the pain receptors, depleting substance P, and making them less sensitive Nicotinic acid/Niacin (Vit. B3) • • • • • • Role: redox cofactor when in form of NAD(P)+/NAD(P)H In animals, produced by degradation of L-Trp In most plants, L-Asp is the precursor Nuc. attack on phosphoglyceraldehyde followed by imine formation Reaction with N-methyl pyrrolinium leads to nicotine Nicotinic acid is also produced during the roasting of coffee from the decomposition of N-methyl derivative trigonelline Structural similarity with acetylcholine leads to stimulant properties Tobacco - the cured and dried leaves of Nicotiana tabacum (Solanaceae) an annual herb indigenous to tropical America, cultivated widely for smoking Tobacco leaves may contain from 0.6–9% of (−)-nicotine (an oily, volatile liquid) together with smaller amounts of structurally related alkaloids. Nicotine in small doses can act as a respiratory stimulant, but in larger doses it causes respiratory depression. Arecoline is the major alkaloid in Areca nuts (betel nuts) - the seeds of Areca catechu (Palmae/Arecaceae), a tall palm cultivated in India & other parts of Asia. Nuts are mixed with lime, wrapped in leaves of the betel pepper and then chewed for their stimulant effect, and subsequent feeling of well-being and mild intoxication. Addition of a C6C2 unit on each side of amine group leads to lobeline Lobelia inflata or “Indian tobacco” contains lobeline, which has a similar but weaker stimulant effect than nicotine Used in smoking deterrent preparations Alkaloids derived from tyrosine, Part 1: Catecholamines Peyote: The dried sliced tops of Lophophora williamsii, found in Mexico & Southwestern US is used to prepare peyote, used by native Americans in religious ceremonies to induce hallucinations. The major psychoactive alkaloid in peyote is mescaline, named after the Mescaleros Apaches. Mechanism of action may involve blocking reuptake of norepinephrine at receptors, increased serotonin release. Cacti have been around since prehistoric times... • Journal of Ethnopharmacology study documented prehistoric peyote use! • El Seedi, et al, 2005 • More info in a separate slideshow. Analyzing ancient alkaloids… • Two peyote buttons (Lophophora) found in a TX cave in 1933 were obtained from Witte museum for the study • Insides were scraped to give a powder that was either extracted with EtOH, basified and further extracted with CHCl3 OR boiled with 10% HCl, extracted with ether. • Dried extracts of 2-14 mg were obtained • Dragendorff test showed alkaloids were present (about 2% by mass) • TLC and GC-MS analysis confirmed mescaline present (isoquinoline alkaloids not found) • Radiocarbon dating analysis showed the samples to be from 37803660 BCE. • Oldest artifact proven to contain a psychoactive substance! (El-Seedi, et al, J. Ethnopharmacology (2005)101: 238-242.) Those wacky cacti! Family: Cactaceae Genus/species: Lophophora williamsii Carnegiea gigantea Many cactus alkaloids have a heterocyclic 6-membered ring (isoquinoline structure): Adrenergic receptors • a-adrenergic receptors: – a1 – blood vessels, causes vasoconstriction – a2 – nerves, mediates synaptic transmission • b-adrenergic receptors: – b1 – heart, cerebral cortex, agonists increase heart muscle contraction – b2 – lungs, cerebellum, agonists dilate bronchi – b3 – adipose tissue, agonists increase lipolysis • General: increases cyclic AMP, activating protein kinases “Beta blockers”: synthetic antagonists of b-adrenergic receptors and some agonists Block b-adrenoreceptors that regulate cardiac muscle contractions (b1) and smooth muscle relaxation in the lungs (b2) Inhibits DOPA decarboxylase cardiac stimulant b1 agonist a1 agonist vasoconstrictor, used in Afrin nasal spray (natural) b1 antagonist controls force of heart contractions, lowers bp a1 antagonist vasodilator lowers bp b2 agonist relaxes bronchial smooth muscle (asthma) Used since 1500’s --CNS Stimulant --Epinephrine Agonist --Bronchodilator --Decongestant --Diet Aid BANNED! Catecholamine mimics from L-Phe The major stimulant in Ephedra is ephedrine, a sympathomimetic amine (similar effects to noradrenaline) Unlike actual catecholamines, ephedrine only acts weakly on receptors, but it can displace noradrenaline from nerve storage vesicles which then stimulates receptors. Ephedrine is orally active and lasts longer than noradrenaline. “Khat” Catha edulis (Celastraceae): In African and Arabian countries, the leaves of this tree are chewed as a stimulant to alleviate hunger and fatigue, while also giving a sensation of general well-being. Users become cheerful and talkative, so khat has become a social drug and use may lead to psychological dependence. Young, fresh leaves are most effective. The principal CNS stimulant is contain (−)cathinone ,with similar pharmacological properties as the synthetic CNS stimulant (+)-amphetamine. Both compounds act by inducing release of catecholamines. Amphetamines usually cause dependence in users. Other amphetamine-like derivatives include MMDA (methoxymethylenedioxyamphet amine) and MDMA (methylenedioxymethamphetamine) MMDA may be formed in the body after ingestion of nutmeg (Myristica fragrans) by an amination process on myristicin, which may account for reported euphoric and hallucinogenic effects of consuming nutmeg.