Presentation - Chem Rxns - stpats-sch3u-sem1-2013
advertisement

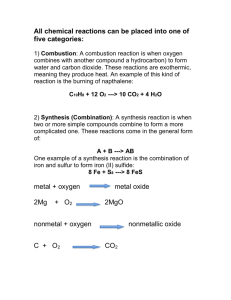
Chemical Reactions SCH3U - Unit 2 Chemical Change Chemical Change = any change in which a new substance is formed Evidence of Chemical Change: Change in colour Change in odour Formation of gas/solid Release/absorption of heat Collision-Reaction Theory A theory stating that chemical reactions involve collisions and rearrangements of atoms or groups of atoms and that the outcome of collisions depends on the energy and orientation of the collisions No reaction occurs if: Molecules don’t have enough energy Molecules don’t collide in the right orientation Orientation The molecules must be in a certain 3-D arrangement to allow a reaction e.g. CH2=CH2 + HCl -> CH3CH2Cl Energy Required A reaction doesn’t occur unless the particles collide with a certain minimum energy called the activation energy of the reaction Activation energy is the minimum energy required before a reaction can occur.You can show this on an energy profile for the reaction. For a simple over-all exothermic reaction, the energy profile looks like this: Activation Energy Profile Chemical Equations Chemical Equation = a representation of a chemical reaction that indicates the: Chemical formulas Relative number of entities States of matter of the reactants and products Reactants Products Chemical Equations In general: Reactant A + Reactant B Product C Reactant = is a chemical that is used up in a chemical reaction Product = is a product that is created during a chemical reaction. Chemical Formulas A chemical formula uses subscripts to indicates the number of atoms in a compound Example: H2O Has 2 atoms of H And 1 atom of O Example: C6H12O6 Has 6 atoms of C Has 12 atoms of H And 6 atoms of O Relative # of Entities Coefficient = a whole number indicating the ratio of molecules of each substance involved in a chemical reaction The large number on the left side of a molecule’s formula Example: Mg + Example: 2 Cl MgCl2 6 K + N2 2 K 3 N State of Matter Solid Liquid Gas Solution = = = = Example: (s) (l) (g) (aq) 6 K(s) + N2(g) 2 K3N(s) Catalysts A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction .e.g conc. H2SO4 in many different reactions Adding a catalyst has exactly this effect on activation energy. A catalyst provides an alternative route for the reaction. That alternative route has a lower activation energy Draw a standard energy profile and then draw a new line to represent the inclusion of a catalyst 5 Types of Chemical Reactions 1. Combustion 2. Synthesis 3. Decomposition 4. Single Displacement 5. Double Displacement 5 Types of Chemical Reactions Generalizations: Combustion: AB + oxygen oxides of A & B + heat Synthesis: A + B C Decomposition: AB A + B Single Displacement: A + BC AC + B Double Displacement: AB + CD AD + CB Combustion Reactions Combustion Reaction: the reaction of a substance with oxygen, producing oxides and energy Also know as burning For a combustion reaction to occur 3 things must be present: 1. 2. 3. Fuel Oxygen Heat Combustion Reactions C Type of Reaction: Synthesis Example C + O2 C + O O O C O C C O O C CC O O C C C C C C CC C General: A + B AB Synthesis Reaction Characteristics Two or more substances (elements or compounds) react to form ONE product. Combination of smaller atoms/molecules into larger molecules. Usually exothermic (energy is produced) Can occur naturally or by an initial application of energy (heat, flame, UV light, use of catalyst) Predicting Products of Synthesis Reactions Metal + oxygen → metal oxide (basic oxide) EX. 2Mg(s) + O2(g) → 2MgO(s) Nonmetal + oxygen → nonmetallic oxide (acidic oxide) EX. C(s) + O2(g) → CO2(g) Metal oxide + water → metallic hydroxide (base) EX. MgO(s) + H2O(l) → Mg(OH)2(s) Nonmetallic oxide + water → acid EX. CO2(g) + H2O(l) → ; H2CO3(aq) Metal + nonmetal → salt EX. 2 Na(s) + Cl2(g) → 2NaCl(s) A few nonmetals combine with each other. EX. 2P(s) + 3Cl2(g) → 2PCl3(g) These two reactions should be remembered: N2(g) + 3H2(g) → 2NH3(g) NH3(g) + H2O(l) → NH4OH(aq) Type of Reaction: Decomposition Example: NaCl Cl Na General: Cl + Na AB A + B Type of Reaction: Decomposition Example 2HgO O Hg O Hg General: Hg + O O Hg AB A + B Decomposition Reaction Characteristics ONE reactant produces two or more products. Splitting of large molecules into elements or smaller molecules. Usually endothermic (requires energy) Can require energy in the form of heat, electricity, catalyst, UV light *some decomposition rxns occur at room temperature Predicting Products of Decomposition Reactions Metallic carbonates, when heated, form metallic oxides and CO2(g). EX. CaCO3(s) → CaO(s) + CO2(g) Most metallic hydroxides, when heated, decompose into metallic oxides and water. EX. Ca(OH)2(s) → CaO(s) + H2O(g) Metallic chlorates, when heated, decompose into metallic chlorides and oxygen. EX. 2KClO3(s) → 2KCl(s) + 3O2(g) Some acids, when heated, decompose into nonmetallic oxides and water. EX. H2SO4 → H2O(l) + SO3(g) Some oxides, when heated, decompose. EX. 2HgO(s) → 2Hg(l) + O2(g) Some decomposition reactions are produced by electricity. EX. 2H2O(l) → 2H2(g) + O2(g) EX. 2NaCl(l) → 2Na(s) + Cl2(g) Type of Reaction: Single Displacement Example: Zn + CuCl2 Cu Cl + Cl General: Zn Zn Cl + Cu Cl AB + C AC + B Type of Reaction: Double Displacement Example: MgO + CaS Mg + O General: Ca S Mg S + Ca O AB + CD AD + CB Chemical Reactions combustion: AB + oxygen oxides of A & B synthesis: A + B C decomposition: AB A + B single displacement: A + BC AC + B double displacement: AB + CD AD + CB