Chapter 8-10 Review - Akron Central Schools
advertisement

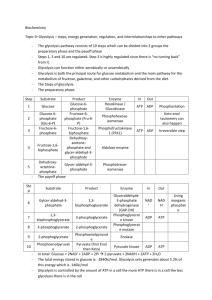
Biological systems use free energy based on empirical data that all organisms require a constant energy input. The first law of thermodynamics states that energy can be neither created nor destroyed. For living organisms, which of the following statements is an important consequence of this first law? • A) The energy content of an organism is constant except for when its cells are dividing. • B) The organism must ultimately obtain all the necessary energy for life from its environment. • C) The entropy of an organism decreases with time as the organism grows in complexity. • D) Organisms are unable to transform energy from the different states in which it can exist. In a biological reaction, succinate dehydrogenase catalyzes the conversion of succinate to fumarate. The reaction is inhibited by malonic acid, a substance that resembles succinate but cannot be acted upon by succinate dehydrogenase. Increasing the amount of succinate molecules to those of malonic acid reduces the inhibitory effect if malonic acid. Select the correct identification of the molecules described in the reaction. • A) Succinate dehydrogenase is the enzyme, and fumarate is the substrate in the reaction. • B) Succinate dehydrogenase is the enzyme, and malonic acid is the substrate in the reaction. • C) Succinate is the substrate, and fumarate is the product in the reaction. • D) Fumarate is the product, and malonic acid is a noncompetitive inhibitor in the reaction. New biosensors, applied like a temporary tattoo to the skin, can alert serious athletes that they are about to "hit the wall" and find it difficult to continue exercising. These biosensors monitor lactate, a form of lactic acid, released in sweat during strenuous exercise. Which of the statements below is the best explanation of why athletes would need to monitor lactate levels? • A) During aerobic respiration, muscle cells cannot produce enough lactate to fuel muscle cell contractions and muscles begin to cramp, thus athletic performance suffers. • B) During anaerobic respiration, lactate levels increase when muscles cells need more energy, however muscles cells eventually fatigue, thus athletes should modify their activities to increase aerobic respiration. • C) During aerobic respiration, muscles cells produce too much lactate which causes a rise in the pH of the muscle cells, thus athletes must consume increased amounts of sports drinks, high in electrolytes, to buffer the pH. • D) During anaerobic respiration, muscle cells receive too little oxygen and begin to convert lactate to pyruvate (pyruvic acid), thus athletes experience cramping and fatigue. ATP synthase is a key enzyme of mitochondrial energy conversion. Mitochondrial ATP synthase deficiency is due to a mutation in a gene important for the formation of a subunit in the ATP synthase complex. Scientists could use cells with this gene mutation to investigate which of the following questions? • A) What effect does the mutation have on the movement of electrons between the electron carriers of the electron transport chain? • B) What effect does the mutation have on the number of protons pumped into the intermembrane space of the mitochondria? • C) What effect does the mutation have on the amount of ATP synthesized during cellular respiration? • D) What effect does the mutation have on the number of water molecules formed at the end of the electron transport chain? The oxidation of glucose in the presence of oxygen involves glycolysis, pyruvate oxidation, and the citric acid cycle (Krebs cycle). Some energy is captured in glycolysis when glucose is converted to pyruvate (pyruvic acid). In the citric acid cycle (Krebs cycle), more energy is captured in the form of . reduced electron carriers, NADH and FADH2 Select the best explanation for why the eventual reoxidation of NADH and FADH2 is crucial for the continuation of the citric acid (Krebs) cycle. • A) In order for the cycle to continue, oxidized electron carriers must be available. • B) Without oxidized electron carriers, oxygen will not accept the electrons at the end of the electron transport chain. • C) Phosphate cannot attach to ADP to form ATP without oxidized electron carriers. • D) The presence of reduced forms of the electron carriers prevents the formation of ATP. Students conducted an experiment to determine the effect of light intensity on the rate of photosynthesis. They punched 40 leaf disks from spinach leaves and used a syringe partially filled with water to pull the gases from the leaf disks so that all leaf disks sunk to the bottom of the syringe. Ten (10) leaf disks from the syringe were placed in each of four cups and covered with 50 ml of the solutions as indicated below. All leaf disks were resting on the bottom of the cups when the experiment began. The volume of liquid in each cup and the temperature of the solutions were held constant. All cups were placed 0.5 meters from the designated light source. A large beaker of water was placed between the light and the cups to act as a heat sink to prevent a change in temperature. At the end of 10 minutes, the number of disks floating in each cup was recorded. Trial Grams of baking soda (CO22 source) Wattage of light bulb Disks floating at 10 minutes 1 0.5 25 3 2 0.5 50 5 3 0.5 75 9 4 0 75 0 Use your knowledge of the mechanism of photosynthesis and the data presented in the chart to determine which of the statements below is a correct explanation for the student's data. • a. Cup 1 had a low rate of photosynthesis because 0.5 grams of baking soda did not provide a sufficient amount of CO2. • b. Cup 2 had the highest rate of photosynthesis because 5 disks were floating at the end of 10 minutes using a 50 watt light bulb. • c. Cup 3 had the same rate of photosynthesis as Cup 1 because they had the same ratio of disks floating to wattage of light. • d. Cup 4 had the slowest rate of photosynthesis because it had the least baking soda. Anabolic pathways ____. • A) are usually highly spontaneous chemical reactions • B) consume energy to build up polymers from monomers • C) release energy as they degrade polymers to monomers • D) consume energy to decrease the entropy of the organism and its environment The mathematical expression for the change in free energy of a system is DG = DH - TDS. Which of the following is (are) correct? • A) DS is the change in enthalpy, a measure of randomness. • B) DH is the change in entropy, the energy available to do work. • C) DG is the change in free energy. • D) T is the temperature in degrees Celsius. Catabolic pathways ____. • A) combine molecules into more energy-rich molecules • B) supply energy, primarily in the form of ATP, for the cell's work • C) are endergonic • D) are spontaneous and do not need enzyme catalysis The active site of an enzyme is the region that ____. • A) binds allosteric regulators of the enzyme • B) is involved in the catalytic reaction of the enzyme • C) binds noncompetitive inhibitors of the enzyme • D) is inhibited by the presence of a coenzyme or a cofactor The following questions are based on the reaction A + B « C + D shown in the accompanying figure. Which of the following terms best describes the forward reaction in the figure? • A) endergonic, DG > 0 • B) exergonic, DG < 0 • C) endergonic, DG < 0 • D) exergonic, DG > 0 Allosteric enzyme regulation is usually associated with ____. • A) feedback inhibition • B) activating activity • C) an enzyme with more than one subunit • D) the need for cofactors Besides turning enzymes on or off, what other means does a cell use to control enzymatic activity? • A) localization of enzymes into specific organelles or membranes • B) exporting enzymes out of the cell • C) connecting enzymes into large aggregates • D) hydrophobic interactions Substrate-level phosphorylation occurs ____. • A) in glycolysis • B) in the citric acid cycle • C) in both glycolysis and the citric acid cycle • D) during oxidative phosphorylation Which of the following statements about NAD+ is true? • A) NAD+ is reduced to NADH during glycolysis, pyruvate oxidation, and the citric acid cycle. • B) NAD+ has more chemical energy than NADH. • C) NAD+ can donate electrons for use in oxidative phosphorylation. • D) In the absence of NAD+, glycolysis can still function. Starting with one molecule of glucose, the energy-containing products of glycolysis are ____. • A) 2 NAD+, 2 pyruvate, and 2 ATP • B) 2 NADH, 2 pyruvate, and 2 ATP • C) 2 FADH2, 2 pyruvate, and 4 ATP • D) 6 CO2, 2 pyruvate, and 2 ATP Which kind of metabolic poison would most directly interfere with glycolysis? • A) an agent that reacts with oxygen and depletes its concentration in the cell • B) an agent that binds to pyruvate and inactivates it • C) an agent that closely mimics the structure of glucose but is not metabolized • D) an agent that reacts with NADH and oxidizes it to NAD+ Most of the CO2 from the catabolism of glucose is released during ____. • A) glycolysis • B) electron transport • C) chemiosmosis • D) the citric acid cycle Carbon dioxide (CO2) is released during which of the following stages of cellular respiration? • A) glycolysis and the oxidation of pyruvate to acetyl CoA • B) oxidation of pyruvate to acetyl CoA and the citric acid cycle • C) oxidative phosphorylation and fermentation • D) fermentation and glycolysis In chemiosmosis, what is the most direct source of energy that is used to convert ADP + i to ATP? • A) energy released as electrons flow through the electron transport system • B) energy released from substrate-level phosphorylation • C) energy released from movement of protons through ATP synthase, down their electrochemical gradient • D) No external source of energy is required because the reaction is exergonic. Energy released by the electron transport chain is used to pump H+ into which location in eukaryotic cells? • A) mitochondrial outer membrane • B) mitochondrial inner membrane • C) mitochondrial intermembrane space • D) mitochondrial matrix Fatty acids usually have an even number of carbons in their structures. They are catabolized by a process called beta-oxidation. The end products of the metabolic pathway are acetyl groups of acetyl CoA molecules. These acetyl groups ____. • A) directly enter the electron transport chain • B) directly enter the energy-yielding stages of glycolysis • C) are directly decarboxylated by pyruvate dehydrogenase • D) directly enter the citric acid cycle The process of photosynthesis probably originated ____. • A) in plants • B) in prokaryotes • C) in fungi • D) three separate times during evolution Refer to the figure. If the carbon atom of each of the incoming CO2 molecules is labeled with a radioactive isotope of carbon, which organic molecules will be radioactively labeled after one cycle? A) C only B) B, C, D, and E C) C, D, and E only D) B and C only Why are C4 plants able to photosynthesize with no apparent photorespiration? • A) They do not participate in the Calvin cycle. • B) They use PEP carboxylase to initially fix CO2. • C) They conserve water more efficiently. • D) They exclude oxygen from their tissues. Referring to the accompanying figure, oxygen would inhibit the CO2 fixation reactions in ____. A) cell I only B) cell II only C) neither cell I nor cell II D) both cell I and cell II