Document
advertisement
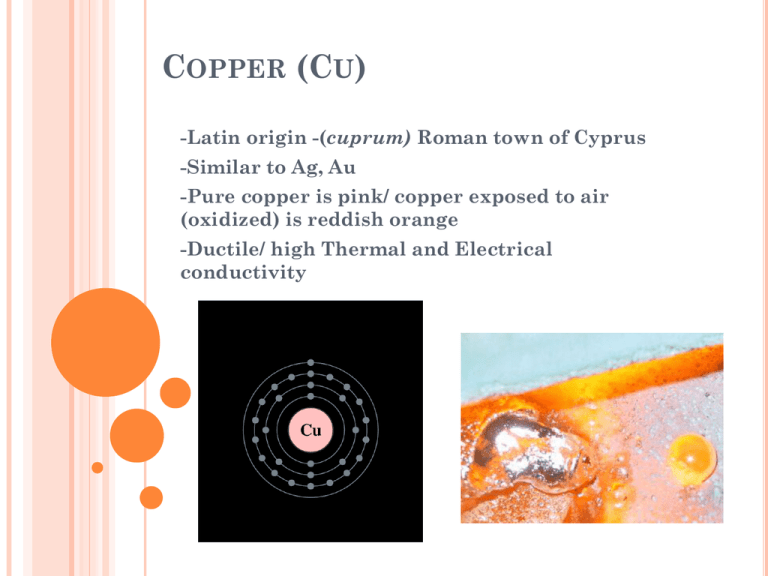
COPPER (CU) -Latin origin -(cuprum) Roman town of Cyprus -Similar to Ag, Au -Pure copper is pink/ copper exposed to air (oxidized) is reddish orange -Ductile/ high Thermal and Electrical conductivity CHEMICAL PROPERTIES Cu+1 (cuprous), Cu+2 (cupric), Cu+3, Cu+4 Water-Soluble Reacts w/ atmospheric Oxygen Copper Corrosion CHEMICAL PROPERTIES Oxygen-containing ammonia solutions give water-soluble complexes with copper Hydrochloric acid/hydrogen peroxide also react with copper chlorides to form copper(II) salts Copper(II) chloride and copper (+0) comproportionate to form copper(I) chloride PRODUCTION HISTORY Most copper (Copper Sulfide) is extracted from large open pit mines Crushed ores are subjected to froth flotation or bioleaching Heating the material with silica removes the iron slag and drops the copper matte to the bottom The copper matte is roasted to oxidize the sulfides The resulting blister copper is heated and blown with natural gas to remove oxygen Electro-refining (electro-platting) the im-pure copper produces pure copper PRODUCTION HISTORY Copper sulfides Copper carbonates Copper Oxides USES AND APPLICATIONS Bronze Age- (Alloying of copper with zinc or tin to make brass and bronze) right after the Chalcolithic age Currency Construction Electrical Wires Machinery Fungicide Antimicrobial Weapons/Tools Art Sculptures Roofing/Plumbing Wood Preservative Biostatic Property Antibiofouling MODE OF ENTRY INTO AQUATIC ENVIRONMENT Copper Water Pipes Contaminated Drinking water (excess CuS) Runoff ladened w/excess CuS sprayed on fruits and vegetables REACTIVITY W/ WATER AND OTHER PROP. Dissolved in Water In form of salts Cu+3 and +4 form fluoride complexes o29 Isotopes of copper •63Cu,65Cu are stable (63Cu 69% of naturally occurring) •67Cu and above,64Cu and below are very unstable •68mCu (3.8 min half-life) o62Cu and 64Cu have significant applications. 64Cu used in X-ray imaging and treating cancer TOXICITY TO AQUATIC LIFE Copper strongly adsorbs into organic matter making it an effective algaecide At acute toxic levels, copper effects fish, invertebrates, and amphibians equally The deleterious effects of copper are seen more commonly in the organs of aquatic organisms than terrestrial organisms mollusks have a higher potential to bioconcentrate copper than do fish effects on bird growth rates and egg production Requires high concentrations to effect mammals liver cirrhosis, kidney necrosis, brain necrosis, and even fetal mortality can occur MODES OF TOXICITY Essential in hemocyanin and cytochrome c oxidase in aerobic respiration Acute toxic levels enter the organism through ingestion from food or water Free copper causes toxicity as it generates reactive oxygen species; superoxide, hydrogen peroxide, the hydroxyl radical These damage proteins, lipids and DNA MODES OF TOXICITY Redox Cycling of Cu(II) in the body Cu(II) strongly catalyzes the oxidation of TBHQ to TBQ TBQH comes from BHA; a food preservative and possible antioxidant However, oxidation of TBQH produces reactive oxidative species H(2)O(2) Leads to extensive DNA strand breaks butylated hydroxyanisole (BHA) 2-tert-butyl(1,4)hydroquinone (TBHQ) 2-tert-butyl(1,4)paraquinone (TBQ) BIOCHEMICAL METABOLISM Alterations in the levels of glycerol, phospholipids, glycerides, sterols, sterol esters and free fatty acids due to copper sulphate treatment in mantle and digestive gland of mollusc Possible mechanism of detoxification, prevalent in this fresh water mollusc Mammals have efficient mechanisms to regulate copper such that they are generally protected from excess dietary copper levels MODES OF DETOXIFICATION Metallothionein Localized in the Golgi apparatus and a cysteine-rich protein Capacity to bind heavy metals through the thiol group of its cysteine residues Provides regulation of physiological heavy metals (Cu, Zn) Therefore, may protect against oxidative stress COPPER AS A POSSIBLE DETOXIFIER OF HALOGENATED COMPOUNDS Fenton's reaction for degradation of perchloroethene (volatile organic compound) Copper accelerates the reaction of iron (III)with hydrogen peroxide to generate increased amounts of hydroxyl and superoxide radicals These radicals can react with a variety of VOCs and mineralize them Enabling targeted VOC extraction from effected areas BIBLIOGRAPHY Slide 1: Inorganic Chemistry. San Diego: Academic Press; 2001 S2: Inorganic Chemistry. San Diego: Academic Press; 2001 S3: Nature's building blocks: an A-Z guide to the elements., Oxford University Press. pp. 121–125; 2011 S4: Chemistry of the Elements., 2nd ed. Oxford; 1997 S5: The Elements, in Handbook of Chemistry and Physics 81st edition. S6: Encyclopedia of the History of Technology. London ; New York: Routledge. pp. 13;48–66 S7: Copper Toxicity., The Eck Institute of Applied Nutrition and Bioenergetics; 1999 S8: Evaluation of Nuclear and Decay Properties., Nuclear Physics A., Atomic Mass Data Center; 729 S9: Principles of bioinorganic chemistry., University Science Books:1994 S10: DNA damage caused by reactive oxygen species originating from a copper-dependent oxidation of the 2-hydroxy catechol of estradiol. Carcinogenesis 15 (7): 1421–142 S11: Copper redox-dependent activation of 2-tert-butyl(1,4)hydroquinone: formation of reactive oxygen species and induction of oxidative DNA damage in isolated DNA and cultured rat hepatocytes. Mutat Res. 2002 Jul 25;518(2):123-33 S12: SUS Environmental Protection Agency., http://www.epa.gov/pesticides/factsheets/copper-alloy-products.htm S13: Metallothioneins and Related Chelators. Metal Ions in Life Sciences. 5. Cambridge: RSC Publishing., Sigel, A.; Sigel, H.; Sigel, R.K.O., ed (2009) S14: A possible mechanism of detoxification of copper, in the fresh water mollusc, Lymnaea luteolaPhysiol Pharmacol. 1993 Oct-Dec





