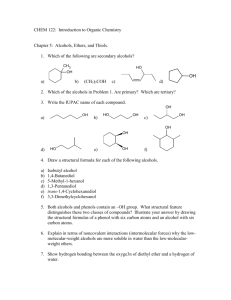
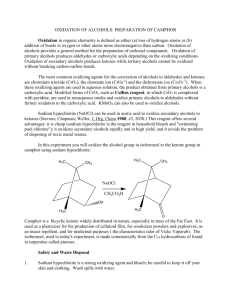
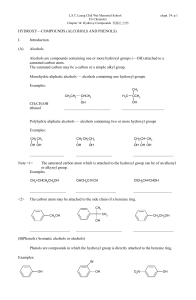
alcohols-II-12-ques
advertisement

Alcohol Reactions Alcohol Reactions Conversions to esters (Acetate Lab) Reactions with hydrogen halides Acid-catalyzed hydrations Esterification Esterification O ROH + O H+ R'COR + R'COH condensation Fischer esterification acid catalyzed reversible H2O Example of Fischer Esterification O COH + CH3OH 0.1 mol 0.6 mol H2SO4 O COCH3 + H2O 70% yield based on benzoic acid Reaction of Alcohols with Acyl Chlorides O ROH + R'CCl O R'COR + High yields Not reversible when carried out in presence of pyridine. HCl Example CH3CH2 O OH + O2N CCl CH3 pyridine CH3CH2 O NO2 OC CH3 (63%) Reaction of Alcohols with Acid Anhydrides O O ROH + R'COCR' O R'COR + analogous to acyl chlorides O R'COH Example O O C6H5CH2CH2OH + F3CCOCCF3 pyridine O C6H5CH2CH2OCCF3 (83%) Reactions of Alcohols “Poor” Reactions of Alcohols Oxidation Conversion to ethers Oxidation of Alcohols Oxidation of Alcohols Primary alcohols O O RCH2OH RCH RCOH Secondary alcohols OH O RCHR' RCR' from H2O Strong Oxidizing Agents In aqueous solution: Mn(VII) Cr(VI) KMnO4 CrO3/ H2SO4 = H2CrO4 K2Cr2O7 / H2SO4 = H2Cr2O7 Oxidize 1o alcohols to carboxylic acids. Cannot stop at aldehydes. Aqueous Cr(VI) H FCH2CH2CH2CH2OH OH K2Cr2O7 H2SO4 H2O Na2Cr2O7 H2SO4 H2O O O FCH2CH2CH2COH (74%) (85%) Question • Treatment of 1-propanol with K2Cr2O7, H2SO4, and heat will produce: • A) • C) B) D) Question What is the product of the following reaction? OH H2CrO4, acetone 35o C OH A. C. O O O OH B. O O H O OH D. O Specialized Oxidizing Agents [Nonaqueous Sources of Cr(VI)] Used in CH2Cl2 Pyridinium dichromate (PDC) (C5H5NH+)2 Cr2O72– Pyridinium chlorochromate (PCC) C5H5NH+ ClCrO3– “Collins Reagent” Jones Reagent: CrO3/ H2SO4 acetone (does not affect Carbon=Carbon double bonds; oxidizes 2o alcohols to ketones) Example: Oxidation of a Primary Alcohol with PCC ClCrO3– +N H PCC CH3(CH2)5CH2OH O CH3(CH2)5CH CH2Cl2 (78%) Question • What is the product of the reaction of 1butanol with PCC in CH2Cl2? • A) B) • C) D) Question What is the product of the following reaction? OH xs PCC, CH2Cl2, 25o C OH A. C. O O O OH B. O O H O OH D. O Example: Oxidation of a Primary Alcohol with PDC (CH3)3C CH2OH PDC CH2Cl2 O (CH3)3C CH (94%) Question • • • • • For the following reaction, select the statement that best describes it. RCH2OH + PDC [(C5H5NH+)2 Cr2O72–] A) The alcohol is oxidized to an acid, and the Cr(VI) is reduced. B) The alcohol is oxidized to an aldehyde, and the Cr(VI) is reduced. C) The alcohol is reduced to an aldehyde, and the Cr(III) is oxidized. D) The alcohol is oxidized to a ketone, and the Cr(VI) is reduced. Conversion of Alcohols to Ethers Conversion of Alcohols to Ethers RCH2O CH2R H OH H+ RCH2O CH2R + H OH Acid-catalyzed Referred to as a "condensation" Equilibrium; most favorable for primary alcohols Example 2CH3CH2CH2CH2OH H2SO4, 130°C CH3CH2CH2CH2OCH2CH2CH2CH3 (60%) Intramolecular Analogue HOCH2CH2CH2CH2CH2OH via: H2SO4 130° •• •• O O (76%) H Reaction normally works well only for 5- and 6-membered rings. H O •• + H Question • When 1-propanol is treated with Na2Cr2O7/H2SO4 followed by treatment with CH3OH, H2SO4 the product isolated is: • A) propanal B) propanoic acid • C) propanol D) methyl propanoate Question Classify the following as oxidation, reduction or neither oxidation or reduction I. Oxidation II. Reduction III. Neither oxidation or reduction A. B. C. D. E. I: a, c, d; II: e, f; III: b I: a, d, e; II: c, f; III: b I: c, d, e; II: a, f; III: b I: c, f; II: a, d, e; III: b I: c, e;II: a, d, f; III: b