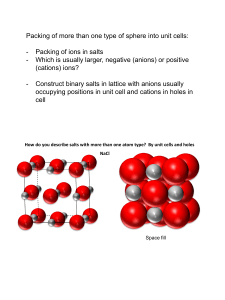
c - Science
advertisement

Intermolecular Forces: Liquids and Solids ● Phases and Phase Diagrams ● Liquids and Liquid Properties ● Intermolecular Forces ● Heating Curves ● Introduction to Solids ● Cubic Packing Arrangements ● Closest-Packed Structures ● Density of a Crystalline Solid ● Ionic Solids and Interstitial Sites ● The Born-Haber Cycle Intermolecular Forces: Liquids and Solids ● Phases and Phase Diagrams ● Liquids and Liquid Properties ● Intermolecular Forces ● Heating Curves ● Introduction to Solids ● Cubic Packing Arrangements ● Closest-Packed Structures ● Density of a Crystalline Solid ● Ionic Solids and Interstitial Sites ● The Born-Haber Cycle Phases and Phase Diagrams very compressible d ≈ 1 – 10 g L−1 at SATP condensation vaporization deposition sublimation fusion incompressible d ≈ 1 – 10 g mL−1 freezing Phases and Phase Diagrams A few definitions: STP vs SATP STP = Standard Temperature and Pressure (0 oC, 100 kPa) SATP = Standard Ambient Temperature and Pressure (25 oC, 100 kPa) 100 kPa = 1 bar Phases and Phase Diagrams Differences between the different states of Matter: Gas: Molecules move randomly and the intermolecular separations are large (i.e. most of a gas is empty space). Liquids and Solids: the molecular motions are quite restricted and the intermolecular separations are small. Solids: The molecules are often, but not always, arranged in regular, repeating patterns. Substances exist in different phases and phase changes occur because molecules exert forces on each other. (Without intermolecular forces, all substances would behave as ideal gases!!) It takes energy to overcome the attractive intermolecular forces that cause molecules to aggregate. Therefore, sublimation, fusion and evaporation are all endothermic processes A given substance will exist as a solid, liquid or gas depending on the temperature and pressure of the sample. A phase diagram shows the stable phases at each temperature and pressure. Phases and Phase Diagrams Phase diagram of I2 Phases and Phase Diagrams Take note of the following points: 1. Solid is the most stable phase at low T and high P. Gas is the stable phase at high T and low P. Phases and Phase Diagrams Take note of the following points: 2. The S-L line shows the T’s and P’s at which both solid and liquid are stable and can coexist. It also shows us how the melting temperature changes with pressure. Phases and Phase Diagrams Take note of the following points: 3. The L-G line curve shows the T’s and P’s at which both liquid and gas are stable and can coexist. It also shows us how the boiling temperature changes with pressure. Phases and Phase Diagrams Take note of the following points: 4. For most substances, the S-L line has a positive slope, but for a few substances (most notably, water but also bismuth and antimony), it has a negative slope! For most substances Phases and Phase Diagrams Take note of the following points: 4. For most substances, the S-L line has a positive slope, but for a few substances (most notably, water but also bismuth and antimony), it has a negative slope! For water Phases and Phase Diagrams Take note of the following points: 5. At the triple point, all three phases are stable and coexist. Phases and Phase Diagrams Take note of the following points: 6. The G-L line ends abruptly at the critical point (Tc, Pc) Phases and Phase Diagrams Phase diagram of I2 What is the phase of I2 at 25 oC and 1 atm? We are dealing with a solid. 25 oC and 1 atm 25 oC Phases and Phase Diagrams Phase diagram of CO2 What is the phase of CO2 at 25 oC and 1 atm? We are dealing with a gas. 1 atm 25 oC and 1 atm 25 oC Phases and Phase Diagrams Phase diagram of H2O What is the phase of H2O at 25 oC and 1 atm? We are dealing with a liquid. 25 oC and 1 atm 1 atm 25 oC Phases and Phase Diagrams Take note of the following points: 4. For most substances, the S-L line has a positive slope, but for a few substances (most notably, water but also bismuth and antimony), it has a negative slope! The slope of the S-L line is negative! Phases and Phase Diagrams Take note of the following points: 1m 1m A column of water 1 m ×1 m × 10 m occupies a volume of 10 m3 or 10,000 L. 1 L of water weighs 1 kg. 10,000 L of water weigh 10,000 kg. The pressure exerted by 10,000 kg of water equals: 9.8 m2/s×10,000 kg/(1 m×1 m) 105 Pa 1 atm 10 m 10 m of water generates a 1 atm additional pressure. Phases and Phase Diagrams Take note of the following points: We find liquid water at the bottom of the ocean. The slope of the S-L line is negative! Phases and Phase Diagrams Take note of the following points: Polymorphism: The existence of a solid substance in more than one form. Other forms of ice obtained at several thousands of atmospheres Phases and Phase Diagrams To summarize, a typical phase diagram looks like this: supercritical solid-liquid coexistence line fluid Solid P critical point B Pc Liquid A 1 atm liquid-vapour coexistence line “normal” melting point Gas Tfus “normal” boiling point Tvap triple point T Tc Phases and Phase Diagrams Supercritical fluid: P Pc solid-liquid coexistence line supercritical fluid (S) B (L) (G) critical point As one moves from A to B, the pressure increases and the density of the gas increases until it equals the density of the liquid. At this point, gas and liquid are indistinguishable, the interface between liquid and gas vanishes and we have a supercritical fluid. A liquid-vapour coexistence line Tc T If a gas is at T > TC (Point A in diagram), increasing the pressure of the gas does not yield a liquid but rather a supercritical fluid (Point B). To take a gas at T > TC and transform it into a liquid, the temperature must first be reduced below TC. Then the pressure is increased to pass the liquid-vapor coexistence curve. Phases and Phase Diagrams P Pc solid-liquid coexistence line supercritical fluid (S) B (L) (G) critical point A liquid-vapour coexistence line Tc T The phase boundary between liquid benzene and its vapour disappears at Tc. T increases from below TC to above TC Supercritical fluid: Below Tc, the phase boundary is clearly visible. Just below Tc, the phase boundary is barely visible. At Tc, the phase boundary disappears. Phases and Phase Diagrams Supercritical fluid: P solid-liquid coexistence line supercritical fluid critical point (S) Pc (L) liquid-vapour coexistence line (G) Tc T Did you know? Supercritical CO2 is used to extract caffeine from coffee beans. The extracted caffeine can be sold to pharmaceutical or beverage companies. The critical point for CO2 is fairly low (Tc = 31 oC) and so, supercritical CO2 can be used at ambient temperatures without causing decomposition or “denaturing” of other compounds. Because it has low toxicity, a low critical temperature and is nonflammable, supercritical CO2 is becoming an increasingly important industrial and commercial solvent. Phases and Phase Diagrams Examples: For a particular substance, the S-L coexistence curve has a negative slope. P solid-liquid coexistence line supercritical fluid critical point (S) Pc (L) PT liquid-vapour coexistence line (G) TT Tc T a) What phase changes are possible if the pressure is increased at constant temperature T? Assume that T is less than Ttp, where Ttp is the triple point temperature. Gas deposition solid melting liquid Phases and Phase Diagrams Examples: For a particular substance, the S-L coexistence curve has a negative slope. P solid-liquid coexistence line supercritical fluid critical point (S) Pc (L) PT liquid-vapour coexistence line (G) TT Tc T b) What phase changes are possible if the pressure is increased at constant temperature T, assuming Ttp < T < Tc, where Ttp and Tc are the triple point and critical point temperatures, respectively. Gas condensation liquid Phases and Phase Diagrams Examples: For a particular substance, the S-L coexistence curve has a negative slope. P solid-liquid coexistence line supercritical fluid critical point (S) Pc (L) PT liquid-vapour coexistence line (G) TT Tc T c) True or False? The melting temperature increases as the pressure increases. False Phases and Phase Diagrams Examples: For a particular substance, the S-L coexistence curve has a negative slope. P solid-liquid coexistence line supercritical fluid critical point (S) Pc (L) PT liquid-vapour coexistence line (G) TT Tc T d) True or False? The solid is more dense than the liquid. At a given temperature, when we increase the pressure, the density increases and the solid becomes a liquid. False Phases and Phase Diagrams Examples: For a particular substance, the triple point is at 57 ºC and 5.1 atm, and the critical point is at 31oC and 73 atm. 73 atm 25 oC and 73 atm 25 oC a) What is the phase of this substance at 25oC and 73 atm? We are dealing with a liquid. Phases and Phase Diagrams Examples: For a particular substance, the triple point is at 57 ºC and 5.1 atm, and the critical point is at 31oC and 73 atm. -60 oC and 75 atm -60 oC and 0.001 atm -60 oC b) What phase changes occur if the pressure is decreased from 75 atm to 0.001 atm at −60 oC? Assume that the solid-liquid line has a positive slope. Solid sublimation gas REVIEW Phases and Phase Diagrams very compressible d ≈ 1 – 10 g L−1 at SATP condensation vaporization deposition sublimation fusion incompressible d ≈ 1 – 10 g mL−1 freezing REVIEW Phases and Phase Diagrams To summarize, a typical phase diagram looks like this: P supercritical fluid Solid Liquid Gas T REVIEW Phases and Phase Diagrams To summarize, a typical phase diagram looks like this: supercritical solid-liquid coexistence line fluid Solid P critical point B Pc Liquid A 1 atm liquid-vapour coexistence line “normal” melting point Gas Tfus “normal” boiling point Tvap triple point T Tc Phases and Phase Diagrams Examples 12-45: Which substances listed in the table can exist as liquids at room temperature (~ 20 oC)? Substance Tc, K Pc, atm H2 33.3 12.8 N2 126.2 33.5 O2 154.8 50.1 CH4 191.1 45.8 CO2 304.2 72.9 HCl 324.6 82.1 NH3 405.7 112.5 SO2 431.0 77.7 H2O 647.3 218.3 P solid-liquid coexistence line supercritical fluid critical point (S) Pc (L) (G) T = 20 oC Tc liquid-vapour coexistence line T Phases and Phase Diagrams Examples 12-45: Which substances listed in the table can exist as liquids at room temperature (~ 20 oC)? Substance Tc, K Pc, atm H2 33.3 12.8 N2 126.2 33.5 O2 154.8 50.1 CH4 191.1 45.8 CO2 304.2 72.9 HCl 324.6 82.1 NH3 405.7 112.5 SO2 431.0 77.7 H2O 647.3 218.3 P solid-liquid coexistence line supercritical fluid critical point (S) Pc (L) (G) liquid-vapour coexistence line T = 20 oCTc = 240 oC Phases and Phase Diagrams Examples 12-45: Which substances listed in the table can exist as liquids at room temperature (~ 20 oC)? Substance Tc, K Pc, atm H2 33.3 12.8 N2 126.2 33.5 O2 154.8 50.1 CH4 191.1 45.8 CO2 304.2 72.9 HCl 324.6 82.1 NH3 405.7 112.5 SO2 431.0 77.7 H2O 647.3 218.3 P solid-liquid coexistence line supercritical fluid critical point (S) Pc (L) (G) T = 20 oC Tc liquid-vapour coexistence line T 20 oC < TC or TC > 293.15 K Gases that can be liquified at room temperature are said to be “non-permanent gases”. Gases that cannot be liquified at room temperature are said to be “permanent gases”. Phases and Phase Diagrams Examples 12-51: Phase diagram of phosphorous a) Indicate the phases present in the regions labeled with a question mark? P (L) ? (S) 43 atm (G) ? 590 oC T Phases and Phase Diagrams Examples 12-51: Phase diagram of phosphorous b) A sample of solid red phosphorous cannot be melted by heating in a container open to the atmosphere. Explain why this is so? P (L) (S) 43 atm (G) 1 atm 590 oC T Solid phosphorous can only be sublimed (S G) if it is heated at P = 1 atm. Phases and Phase Diagrams Examples 12-51: Phase diagram of phosphorous c) Trace the phase changes that occur when the pressure on a sample is reduced from Point A to B, at constant temperature. P A (L) (S) 43 atm B (G) 590 oC Solid condensation Liquid vaporization Gas T Intermolecular Forces: Liquids and Solids ● Phases and Phase Diagrams ● Liquids and Liquid Properties ● Intermolecular Forces ● Heating Curves ● Introduction to Solids ● Cubic Packing Arrangements ● Closest-Packed Structures ● Density of a Crystalline Solid ● Ionic Solids and Interstitial Sites ● The Born-Haber Cycle Intermolecular Forces: Liquids and Solids ● Phases and Phase Diagrams ● Liquids and Liquid Properties ● Intermolecular Forces ● Heating Curves ● Introduction to Solids ● Cubic Packing Arrangements ● Closest-Packed Structures ● Density of a Crystalline Solid ● Ionic Solids and Interstitial Sites ● The Born-Haber Cycle Liquids and Liquid Properties Condensation P solid-liquid coexistence line supercritical fluid critical point (S) (L) (G) liquid-vapour coexistence line T We know that if the temperature of a gas is lowered sufficiently, the gas will condense to a liquid. Why is this? As T is lowered, the average kinetic energy of the molecules decreases. At some point, the molecules will no longer have enough kinetic energy to overcome the attractive forces that draw the molecules together. Consequently, the molecules cluster together to form a liquid. Liquids and Liquid Properties Freezing P solid-liquid coexistence line supercritical fluid critical point (S) (L) (G) liquid-vapour coexistence line T The freezing of a liquid can be explained in the same way: If the temperature of a liquid is lowered sufficiently, the molecules will not have enough kinetic energy to overcome attractive forces that draw the molecules closer together the liquid freezes. Liquids and Liquid Properties The physical properties of a liquid depend on the strength and nature of the intermolecular forces. We shall examine why the following physical properties are different from substance to substance. vapour pressure = equilibrium pressure of vapour that forms above a liquid in a closed container normal boiling point (Tvap) = temperature at which the vapour pressure of the liquid equals 1 atm surface tension (g) = energy required to increase the surface area of a liquid viscosity (η) provides a measure of a fluid’s resistance to flow; the speed of flow through a tube is inversely proportional to the viscosity In general, the stronger the intermolecular attractions, the higher the boiling point, the greater the surface tension, the higher the viscosity and the lower the vapour pressure. Liquids and Liquid Properties vapour pressure = equilibrium pressure of vapour that forms above a liquid in a closed container to vacuum air liquid Liquids and Liquid Properties vapour pressure = equilibrium pressure of vapour that forms above a liquid in a closed container to vacuum air liquid Liquid N2 Liquids and Liquid Properties vapour pressure = equilibrium pressure of vapour that forms above a liquid in a closed container to vacuum air solid Liquid N2 Liquids and Liquid Properties vapour pressure = equilibrium pressure of vapour that forms above a liquid in a closed container to vacuum vacuum solid Liquid N2 Liquids and Liquid Properties vapour pressure = equilibrium pressure of vapour that forms above a liquid in a closed container to vacuum vacuum solid Liquid N2 Liquids and Liquid Properties vapour pressure = equilibrium pressure of vapour that forms above a liquid in a closed container to vacuum vacuum solid Liquids and Liquid Properties vapour pressure = equilibrium pressure of vapour that forms above a liquid in a closed container to vacuum vacuum liquid Liquids and Liquid Properties vapour pressure = equilibrium pressure of vapour that forms above a liquid in a closed container to vacuum liquid Liquids and Liquid Properties The physical properties of a liquid depend on the strength and nature of the intermolecular forces. We shall examine why the following physical properties are different from substance to substance. vapour pressure = equilibrium pressure of vapour that forms above a liquid in a closed container normal boiling point (Tvap) = temperature at which the vapour pressure of the liquid equals 1 atm surface tension (g) = energy required to increase the surface area of a liquid viscosity (η) provides a measure of a fluid’s resistance to flow; the speed of flow through a tube is inversely proportional to the viscosity In general, the stronger the intermolecular attractions, the higher the boiling point, the greater the surface tension, the higher the viscosity and the lower the vapour pressure. Liquids and Liquid Properties Clausius-Clapeyron equation P solid-liquid coexistence line supercritical fluid critical point (S) (L) (G) liquid-vapour coexistence line T The L-G line shows us how 1. the vapour pressure of a liquid changes with temperature 2. the boiling temperature of a liquid changes with pressure Liquids and Liquid Properties Clausius-Clapeyron equation P solid-liquid coexistence line supercritical fluid critical point (S) (L) (G) liquid-vapour coexistence line T Along the L-G line, both liquid and gas co-exist. At equilibrium, the rate of evaporation equals the rate of condensation Vapour X(g) Liquid X(l) Liquids and Liquid Properties Clausius-Clapeyron equation The variation of vapour pressure with temperature is modeled reasonably well by the Clausius-Clapeyron equation: P ln 2 P1 H vap 1 o R T2 1 T1 The quantities appearing in this equation are described below. P2 P1 o H vap R = = vapour pressure at temperature T2 vapour pressure at temperature T1 = = standard enthalpy of vaporization 8.3145 J K−1 mol−1 Extremely important: Pay attention to the units! Liquids and Liquid Properties Clausius-Clapeyron equation P supercritical solid-liquid coexistence line fluid critical point (S) (L) liquid-vapour coexistence line (P2, T2) (P1, T1) (G) T P ln 2 P1 H vap 1 o R T2 1 T1 Liquids and Liquid Properties Clausius-Clapeyron equation P supercritical solid-liquid coexistence line fluid critical point (S) (L) (P2, T2) (P1, T1) liquid-vapour coexistence line (G) T Example: a) If the vapour pressure of P4(l) is 10 Torr at 128 oC and 400 Torr at 251 oC, then what is vapHo? vap H o 1 1 P2 Ln P R 1 T2 T1 P 10 RLn 2 8.3145Ln P 400 1 Ho 52.4 kJ / mol vap H o vap 1 1 1 1 128 273.15 251 273.15 T2 T1 Liquids and Liquid Properties P1 = 10 Torr T1 = 128 oC P2 = 400 Torr T2 = 251 oC vapHo = 52.4 kJ/mol Clausius-Clapeyron equation Example: b) What is the normal boiling point of P4(l)? vap H o 1 1 P2 Ln R T2 T1 P1 P2 1 1 R Ln o vap H P1 T2 T1 T2 T2 P2 1 1 R Ln T2 T1 vap H o P1 1 P2 1 R Ln o T1 vap H P1 1 553.8 K 1 8.3145 760 Ln 128 273.15 52,400 10 Liquids and Liquid Properties Clausius-Clapeyron equation Example: c) What is the vapour pressure at 200 oC? vap H o 1 1 P2 Ln P R 1 T2 T1 vap H o 1 1 P2 exp P1 R T2 T1 P1 = 10 Torr T1 = 128 oC P2 = 400 Torr T2 = 251 oC vapHo = 52.4 kJ/mol vap H o P2 P1 exp R 52,400 1 1 P2 10 exp 109 torr 8 . 3145 200 273 . 15 128 273 . 15 1 1 T2 T1 REVIEW Liquids and Liquid Properties The physical properties of a liquid depend on the strength and nature of the intermolecular forces. We shall examine why the following physical properties are different from substance to substance. vapour pressure = equilibrium pressure of vapour that forms above a liquid in a closed container normal boiling point (Tvap) = temperature at which the vapour pressure of the liquid equals 1 atm surface tension (g) = energy required to increase the surface area of a liquid viscosity (η) provides a measure of a fluid’s resistance to flow; the speed of flow through a tube is inversely proportional to the viscosity In general, the stronger the intermolecular attractions, the higher the boiling point, the greater the surface tension, the higher the viscosity and the lower the vapour pressure. Intermolecular Forces: Liquids and Solids ● Phases and Phase Diagrams ● Liquids and Liquid Properties ● Intermolecular Forces ● Heating Curves ● Introduction to Solids ● Cubic Packing Arrangements ● Closest-Packed Structures ● Density of a Crystalline Solid ● Ionic Solids and Interstitial Sites ● The Born-Haber Cycle Intermolecular Forces Dipole moments and polarizabilities of a few molecules. (Based on data from Physical Chemistry, 6th Edition, by P. Atkins, published by Freeman, 1998). The purpose of this section is to understand how molecules interact with each other and how these interactions help us to understand trends in the physical properties of compounds (e.g. boiling points, vapour pressures, viscosity and surface tensions of liquids; densities and melting points of solids; deviations from ideal gas behaviour, etc.) Generally speaking, differences in the physical properties of substances can often be rationalized by considering how molecules interact with each other at the molecular level. We’ll look at some of the types of intermolecular forces that act between pairs of molecules. μ (in α/αHe debye) H2 0 4.1 HF 1.91 2.6 HCl 1.08 13 HBr 0.80 18 CO 0.12 10 CO2 0 13 H2O 1.85 7.5 NH3 1.47 11 He 0 1 Ar 0 8.4 CH4 0 13 CCl4 0 53 Intermolecular Forces A. Dipole-dipole forces Some molecules possess a permanent dipole moment, μ, because the bond dipoles do not cancel out. Such molecules are said to be “polar” because one end of the molecule is slightly positive and the other end is slightly negative. The charge distribution of a polar molecule can be represented by an arrow that points from the positive end to the negative end. The dipole moments of a few molecules were given in the previous table. Polar molecules (i.e. dipoles) tend to orient themselves in a “head-to-tail” manner, as shown below: The molecules are not organized into perfect straight line because the molecules are in motion (i.e. each one possesses kinetic energy) and they “jiggle” out of perfect alignment. Digging Deeper For a pair of interacting polar molecules with dipole moments of μA and μB: Potential Energy μA μB d A2 B2 d6 Intermolecular Forces A. Dipole-dipole forces Example: Iodine chloride, ICl, and bromine, Br2, have exactly the same number of electrons, and it is reasonable to assume that these molecules are essentially the same size. Yet the boiling points of ICl(l) and Br2(l) are quite different, 97 oC and 59 oC, respectively. Use your knowledge of dipole-dipole interactions to explain why ICl(l) has a higher boiling point than Br2(l). Intermolecular Forces A. Dipole-dipole forces Example: Iodine chloride, ICl, and bromine, Br2, have exactly the same number of electrons, and it is reasonable to assume that these molecules are essentially the same size. Yet the boiling points of ICl(l) and Br2(l) are quite different, 97 oC and 59 oC, respectively. Use your knowledge of dipole-dipole interactions to explain why ICl(l) has a higher boiling point than Br2(l). Chlorine is more electronegative than bromine. Consequently, I-Cl shows a dipole moment: (d)ICl(d) Br2 on the other hand cannot generate a dipole moment because the molecule is made of two identical bromine atoms. Intermolecular Forces B. London dispersion forces The electrons in a molecule are in constant motion and at any particular instant, there may be an asymmetric distribution of electrons in the molecule (i.e. with a greater number of electrons at one end than at the other end). The asymmetric distribution of electrons gives rise to an instantaneous and temporary dipole moment (inst ). The formation of inst in a molecule causes (or induces) the formation of a dipole in neighbouring molecules. The induced dipole moment is ind . (Notice the head-to-tail arrangement of the instantaneous and induced dipole moments.) Molecule A ---- --- - - -- inst Molecule B ---- --- - - -- ind There is an attraction between inst and ind . The strength of the interaction increases as the “polarizabilities” of the molecules increase. Intermolecular Forces B. London dispersion forces provides a measure of the extent to which the charge cloud of a molecule can be distorted (i.e. polarized) by another molecule. Polarizability (α) The charge cloud of a large molecule is diffuse and easily polarized. The charge cloud of a small, compact molecule is not easily polarized. The polarizabilities of a few molecules were given earlier. Note that the larger the molecule, the larger the polarizability. Digging Deeper For a pair of molecules with polarizabilities of αA and αB: αA αB d Potential Energy A B d6 Intermolecular Forces B. London dispersion forces Remarks: London dispersion forces are most attractive when the molecules are large because large molecules have larger, more diffuse (i.e. more polarizable) charge clouds. London dispersion forces always contribute to the molecular interactions because all molecules have charge clouds and are therefore polarizable to some extent. Molecule A ---- --- - - -- inst Molecule B ---- --ind - - -- Intermolecular Forces B. London dispersion forces μ (in Example: Methane (CH4) and carbon tetrachloride (CCl4) are both nonpolar molecules. Use your knowledge of London dispersion forces to explain why the boiling point of CCl4(l) is much higher than that of CH4(l). The C-Cl bonds are very polar, but the bond dipoles cancel. The CCl4 molecule is nonpolar. Carbon tetrachloride (CCl4) is a molecule that is much larger than methane (CH4). Consequently, CCl4 contains many more electrons which can generate an instantaneous dipole moment more readily than CH4. These dipole moments generate strong intermolecular forces between CCl4 molecules which are more difficult to break than in CH4. Consequently, Tb of CCl4 (78oC) is larger than Tb of CH4 (162 oC). α/αHe debye) H2 0 4.1 HF 1.91 2.6 HCl 1.08 13 HBr 0.80 18 CO 0.12 10 CO2 0 13 H2O 1.85 7.5 NH3 1.47 11 He 0 1 Ar 0 8.4 CH4 0 13 CCl4 0 53 Intermolecular Forces C. Hydrogen bonding forces A special type of bond forms between molecules when the molecules contain a hydrogen atom bonded to N, O, or F. When H is bonded to N, O or F, the H atom carries a significant positive charge and it is strongly attracted to a lone pair on another molecule! When a hydrogen atom which is covalently bonded to one atom is simultaneously attracted to the lone pair on another atom, it is “bridging” two molecules, as shown below. Such a bond is called a hydrogen bond. H X Y An intermolecular hydrogen bond “bridges” two molecules. An intramolecular hydrogen bond bridges two parts of the same molecule. intermolecular hydrogen bond Did you know? “inter” means “between” and “intra” means “within”. Intermolecular Forces C. Hydrogen bonding forces Note carefully: H is covalently bonded to X but is simultaneously attracted to a lone pair of electrons on Y. Both X and Y must be N, O or F Hydrogen bonds are the strongest type of intermolecular force (but they are still weak in comparison to covalent and ionic bonding forces) intermolecular forces H X chemical bonding forces Y dipole-dipole & LDFs H bonds covalent & ionic bonds 0.10-10 kJ mol−1 10−40 kJ mol−1 100’s or 1000’s kJ mol−1 intermolecular hydrogen bond Intermolecular Forces C. Hydrogen bonding forces Hydrogen bonds are important! H bonds between H2O’s in ice give the solid an open structure H bonds between H2O’s in water give the liquid a high BP, high surface tension and a large heat capacity. Hydrogen bonding in water. This is Figure 12-7 of Petrucci 10e. Used with permission. Intermolecular Forces C. Hydrogen bonding forces Hydrogen bonds are important! H bonds are especially important in biology (e.g. H bonds keep the two helices of DNA together; the structures and functions of proteins and enzymes are determined by H bonds) The helical structures of proteins (above) and DNA (on the right) are stabilized by hydrogen bonds. These are Figures 28-12 and 28-26 of Petrucci 10e. Used with permission. Intermolecular Forces C. Hydrogen bonding forces Hydrogen bonds are important! H bonds are especially important in biology (e.g. H bonds keep the two helices of DNA together; the structures and functions of proteins and enzymes are determined by H bonds) REVIEW Intermolecular Forces A. Dipole-dipole forces Polar molecules with a dipole moment . B. London dispersion forces The formation of inst in a molecule causes (or induces) the formation of a dipole in neighbouring molecules. Molecule A ---- --- - - -- inst Molecule B ---- --- - - -- ind C. Hydrogen bonding forces A special type of bond forms between molecules when the molecules contain a hydrogen atom bonded to N, O, or F. intermolecular hydrogen bond H X Y Intermolecular Forces C. Hydrogen bonding forces A special type of bond forms between molecules when the molecules contain a hydrogen atom bonded to N, O, or F. When H is bonded to N, O or F, the H atom carries a significant positive charge and it is strongly attracted to a lone pair on another molecule! intermolecular hydrogen bond H X Y Electronegativity Scale Intermolecular Forces C. Hydrogen bonding forces Example: Dichloroethene, C2H2Cl2, has several isomeric forms. Use your knowledge of intermolecular forces to predict whether (Z)-1,2-dichloroethene or (E)-1,2-dichloroethene has the higher boiling point. Lewis structures are given below. H H C Tb = 60 oC Cl Cl vs C Cl (Z)-1,2-dichloroethene, also called cis-1,2-dichloroethene H C C Tb = 48 oC H Cl (E)-1,2-dichloroethene, also called trans-1,2-dichloroethene The chlorine atom being more electronegative than carbon induces a dipole moment in the C-Cl bond. However, those dipole moments are opposite in (E)-1,2-dichloroethane and they cancel each other. The dipole moments of the C-Cl bond do not cancel each other in (Z)-1,2-dichloroethane. Thus the Z-isomer has a permanent dipole moment which induces strong intermolecular forces. The Z-isomer has a higher boiling point. A word of warning! Don’t over generalize the results of this example. You might end up making the wrong prediction! Intermolecular Forces C. Hydrogen bonding forces Example: Consider the trans and cis isomers of C4H4O4. Which one has the higher melting point? O O OH HO Tm = 300 oC OH OH O Tm = 140 oC O Fumaric acid (trans) Maleic acid (cis) Fumaric acid generates intermolecular H-bonds which leads to the formation of a network where all the molecules are associated with another. Maleic acid forms intramolecular H-bonds and the intermolecular forces between molecules are weaker. Consequently, maleic acid has a lower melting point than fumaric acid. Intermolecular Forces C. Hydrogen bonding forces Example: Consider the trans and cis isomers of C4H4O4. Which one has the higher melting point? O O OH HO Tm = 300 oC OH OH O Tm = 140 oC O Fumaric acid (trans) Maleic acid (cis) Fumaric acid generates intermolecular H-bonds which leads to the formation of a network where all the molecules are associated with another. Maleic acid forms intramolecular H-bonds and the intermolecular forces between molecules are weaker. Consequently, maleic acid has a lower melting point than fumaric acid. Intermolecular Forces C. Hydrogen bonding forces Example: Use your knowledge of intermolecular forces to predict whether CH3COCH3(l) or CH3CH2CH2OH(l) has the higher boiling point. O Tb = 56 oC O H 3C CH3 acetone H Tb = 97 oC 1-propanol Acetone and 1-propanol have a similar number of C- and O-atoms. They both induce a dipole moment since O is more electronegative than C. However, 1-propanol can form H-bonds and acetone cannot. Consequently, acetone boils at a lower temperature than 1-propanol. Intermolecular Forces C. Hydrogen bonding forces Example: Which one of the liquids, HO-CH2CH2-OH or CH3CH2OH, has the highest vapour pressure at room temperature? Tb = 196 oC H O O O Ethylene glycol H H Tb = 78 oC Ethanol A single ethylene glycol (EG) can form more H-bonds with other EG molecules than ethanol can form with other ethanol molecules. Consequently, EG boils at a higher temperature. EG has a lower vapor pressure than ethanol. Intermolecular Forces C. Hydrogen bonding forces Example: Use your knowledge of intermolecular forces to predict the order of boiling points for H2O, H2S (hydrogen sulfide), H2Se (hydrogene selenide), and H2Te (hydrogen telluride). All these molecules are bent, and as such, generate a dipole moment. X H H Intermolecular Forces C. Hydrogen bonding forces Example: Use your knowledge of intermolecular forces to predict the order of boiling points for H2O, H2S, H2Se and H2Te. All these molecules are bent, and as such, generate a dipole moment. X H H Tb 120 100 -59.6 oC -41.3 oC -2 oC 80 T b , oC 60 40 Tb (oC) = 0.6055xM (g/mol) - 83.785 20 0 -20 -40 HO 2 Tb =-6060 oC H2S Tb = 60 oC -80 0 20 40 H2Te Tb = 2 oC H2Se Tb = 41 oC 60 80 100 Mass, g/mol 120 140 160 Intermolecular Forces C. Hydrogen bonding forces Example: Use your knowledge of intermolecular forces to predict the order of boiling points for H2O, H2S, H2Se and H2Te. All these molecules are bent, and as such, generate a dipole moment. X H H Tb +100 oC -59.6 oC -41.3 oC -2 oC 120 H2O Tb = 100 oC 100 80 T b , oC 60 40 Tb (oC) = 0.6055xM (g/mol) - 83.785 20 0 -20 H2S Tb = 60 oC -40 -60 -80 0 20 40 H2Te Tb = 2 oC H2Se Tb = 41 oC 60 80 100 Mass, g/mol 120 140 160 Intermolecular Forces C. Hydrogen bonding forces Example: Use your knowledge of intermolecular forces to predict the order of boiling points for H2O, H2S, H2Se and H2Te. By increasing the mass and the size of the molecule H2X, one increases the polarizability, and thus the strength of the interactions between the H2X molecules. The exception is H2O because water forms H-bonds: H2S < H2Se < H2Te < H2O Tb +100 oC -59.6 oC -41.3 oC -2 oC 120 H2O Tb = 100 oC 100 80 T b , oC 60 40 Tb (oC) = 0.6055xM (g/mol) - 83.785 20 0 -20 H2S Tb = 60 oC -40 -60 -80 0 20 40 H2Te Tb = 2 oC H2Se Tb = 41 oC 60 80 100 Mass, g/mol 120 140 160 Intermolecular Forces C. Hydrogen bonding forces Example: Use your knowledge of intermolecular forces to predict the order of boiling points for H2O, H2S, H2Se and H2Te. It should be noted that similar arguments can be applied to the hydrides of the group 15 and group 17 elements. Thus, in order of increasing BP, we have: PH3 < AsH3 < SbH3 < NH3 and HCl < HBr < HI < HF Intermolecular Forces: Liquids and Solids ● Phases and Phase Diagrams ● Liquids and Liquid Properties ● Intermolecular Forces ● Heating Curves ● Introduction to Solids ● Cubic Packing Arrangements ● Closest-Packed Structures ● Density of a Crystalline Solid ● Ionic Solids and Interstitial Sites ● The Born-Haber Cycle Review this section on your own. Heating Curves A heating curve shows us how the temperature varies with the amount of heat added. Consider heating a sample of ice from ti = –10 oC to t = 150 oC at constant pressure. T warming vapour (slope3) tf boiling liquid tvap warming liquid (slope2) warming solid (slope1) melting solid tfus ti qgas qliq qsol Hfus Hvap Q Intermolecular Forces: Liquids and Solids ● Phases and Phase Diagrams ● Liquids and Liquid Properties ● Intermolecular Forces ● Heating Curves ● Introduction to Solids ● Cubic Packing Arrangements ● Closest-Packed Structures ● Density of a Crystalline Solid ● Ionic Solids and Interstitial Sites ● The Born-Haber Cycle Introduction to Solids Solids can be classified as crystalline or as amorphous, depending on whether we have regular or irregular packing of the atoms, molecules or ions that make up the solid. Crystalline and amorphous solids have rather different physical characteristics. Crystalline solids • regular repeating patterns • “sharp” melting points Amorphous solids • irregular packing • melt over a temperature range Examples of amorphous solids include rubber, polystyrene, window glass, candle wax, and cotton candy. We can get irregular packing (amorphous solids) if there are impurities in the sample when the liquid freezes, or if the molecules are large or have flexible structures. For molecules that are large or very flexible (i.e. polymers!), it is statistically most probable that the molecules will be irregularly packed when the liquid freezes. We shall focus exclusively on crystalline solids. Introduction to Solids For crystalline solids, we use the following concepts to characterize the pattern of the packing arrangement. Crystal lattice three dimensional array of points that shows how the structural units are arranged in space. Each structural unit could be a single atom, a molecule or an ion. In C(s), the structural unit is a carbon atom. In I2(s), the structural unit is an I2 molecule. In NH4NO3(s), the structural units are NH4+ and NO3− ions. Unit cell the smallest building block that possesses the symmetry of the crystal lattice; a solid sample of any size can be “built” by stacking together unit cells. Introduction to Solids Crystalline solids are classified according to the nature of the bonding, or according to the geometry and symmetry of the packing arrangement. Let’s focus first on the nature of the bonding. Crystalline solids ionic Network covalent molecular In an ionic solid, In a network positive and covalent solid, negative ions are atoms are held in held in their lattice their lattice positions positions by (strong) by (strong) covalent ionic bonding bonds. forces. In a molecular solid, molecules are held in their lattice positions by (weak) intermolecular forces. oC H2O Tm = 0 NaCl Tm = 800 SiO2 Tm= 1600 LiF Tm = 848 oC SiC Tm =2830 oC S Tm = 113 oC 8 o ZnO Tm = 1974 C Cdiamond Tm = 4440 oC I2 Tm = 114 oC (at P = 12.4 GPa) oC oC metallic In a metallic solid, metal cations are held in their lattice positions by (strong) metallic bonding forces. Cu Tm = 1083 oC Ag Tm = 962 oC W Tm = 3422 oC Introduction to Solids Crystalline solids are classified according to the nature of the bonding, or according to the geometry and symmetry of the packing arrangement. Let’s focus first on the nature of the bonding. Crystalline solids ionic Network covalent molecular In an ionic solid, In a network positive and covalent solid, negative ions are atoms are held in held in their lattice their lattice positions positions by (strong) by (strong) covalent ionic bonding bonds. forces. In a molecular solid, molecules are held in their lattice positions by (weak) intermolecular forces. oC H2O Tm = 0 NaCl Tm = 800 SiO2 Tm= 1600 LiF Tm = 848 oC SiC Tm =2830 oC S Tm = 113 oC 8 o ZnO Tm = 1974 C Cdiamond Tm = 4440 oC I2 Tm = 114 oC (at P = 12.4 GPa) oC oC metallic In a metallic solid, metal cations are held in their lattice positions by (strong) metallic bonding forces. Cu Tm = 1083 oC Ag Tm = 962 oC W Tm = 3422 oC Introduction to Solids The Periodic Table can help understand the differences in Tm between NaCl, LiF, and ZnO. NaCl Tm = 800 oC 1 q A qC Electrostatic Force LiF Tm = 848 oC 2 4 r o A C ZnO Tm = 1974 oC Introduction to Solids Crystalline solids are classified according to the nature of the bonding, or according to the geometry and symmetry of the packing arrangement. Let’s focus first on the nature of the bonding. Crystalline solids ionic Network covalent molecular In an ionic solid, In a network positive and covalent solid, negative ions are atoms are held in held in their lattice their lattice positions positions by (strong) by (strong) covalent ionic bonding bonds. forces. In a molecular solid, molecules are held in their lattice positions by (weak) intermolecular forces. oC H2O Tm = 0 NaCl Tm = 800 SiO2 Tm= 1600 LiF Tm = 848 oC SiC Tm =2830 oC S Tm = 113 oC 8 o ZnO Tm = 1974 C Cdiamond Tm = 4440 oC I2 Tm = 114 oC (at P = 12.4 GPa) oC oC metallic In a metallic solid, metal cations are held in their lattice positions by (strong) metallic bonding forces. Cu Tm = 1083 oC Ag Tm = 962 oC W Tm = 3422 oC Introduction to Solids Crystalline solids are classified according to the nature of the bonding, or according to the geometry and symmetry of the packing arrangement. Let’s focus first on the nature of the bonding. Crystalline solids ionic Network covalent molecular In an ionic solid, In a network positive and covalent solid, negative ions are atoms are held in held in their lattice their lattice positions positions by (strong) by (strong) covalent ionic bonding bonds. forces. In a molecular solid, molecules are held in their lattice positions by (weak) intermolecular forces. oC H2O Tm = 0 NaCl Tm = 800 SiO2 Tm= 1600 LiF Tm = 848 oC SiC Tm =2830 oC S Tm = 113 oC 8 o ZnO Tm = 1974 C Cdiamond Tm = 4440 oC I2 Tm = 114 oC (at P = 12.4 GPa) oC oC metallic In a metallic solid, metal cations are held in their lattice positions by (strong) metallic bonding forces. Cu Tm = 1083 oC Ag Tm = 962 oC W Tm = 3422 oC Introduction to Solids Crystalline solids are classified according to the nature of the bonding, or according to the geometry and symmetry of the packing arrangement. Let’s focus first on the nature of the bonding. Crystalline solids ionic Network covalent molecular In an ionic solid, In a network positive and covalent solid, negative ions are atoms are held in held in their lattice their lattice positions positions by (strong) by (strong) covalent ionic bonding bonds. forces. In a molecular solid, molecules are held in their lattice positions by (weak) intermolecular forces. oC H2O Tm = 0 NaCl Tm = 800 SiO2 Tm= 1600 LiF Tm = 848 oC SiC Tm =2830 oC S Tm = 113 oC 8 o ZnO Tm = 1974 C Cdiamond Tm = 4440 oC I2 Tm = 114 oC (at P = 12.4 GPa) oC oC metallic In a metallic solid, metal cations are held in their lattice positions by (strong) metallic bonding forces. Cu Tm = 1083 oC Ag Tm = 962 oC W Tm = 3422 oC Introduction to Solids Metallic solids can be viewed as an array of metal cations bathing in a sea of valence electrons. Electron sea model of metallic bonding + + + + + + + + + + + + + + + A “sea” of delocalized valence electrons Introduction to Solids When we focus on the geometry of the packing arrangements, we find that there are seven basic shapes for unit cells. The shape of each unit cell is described in terms of three lengths (a, b and c) and three angles (, , and g). We will focus primarily on cubic unit cells. The others are mentioned only to emphasize that there are other types/shapes of unit cells besides cubic unit cells. cubic a=b=c = = g = 90o trigonal a=b=c tetragonal = = g ≠ 90o a=b≠c hexagonal = = g = 90o a=b≠c o; = = 90 monoclinic and g = 120o a≠b≠c = g = 90o triclinic and ≠ 90o a≠b≠c orthorhombic ≠ ≠ g ≠ 90o g a≠b≠c = = g = 90o a b c Introduction to Solids When we focus on the geometry of the packing arrangements, we find that there are seven basic shapes for unit cells. The shape of each unit cell is described in terms of three lengths (a, b and c) and three angles (, , and g). We will focus primarily on cubic unit cells. The others are mentioned only to emphasize that there are other types/shapes of unit cells besides cubic unit cells. cubic a=b=c = = g = 90o trigonal a=b=c tetragonal = = g ≠ 90o a=b≠c hexagonal = = g = 90o a=b≠c o monoclinic = = 90 ;o and g = 120 a≠b≠c = g = 90o triclinic and ≠ 90o a≠b≠c orthorhombic ≠ ≠ g ≠ 90o a≠b≠c = = g = 90o a a a Intermolecular Forces: Liquids and Solids ● Phases and Phase Diagrams ● Liquids and Liquid Properties ● Intermolecular Forces ● Heating Curves ● Introduction to Solids ● Cubic Packing Arrangements ● Closest-Packed Structures ● Density of a Crystalline Solid ● Ionic Solids and Interstitial Sites ● The Born-Haber Cycle REVIEW Introduction to Solids For crystalline solids, we use the following concepts to characterize the pattern of the packing arrangement. Crystal lattice three dimensional array of points that shows how the structural units are arranged in space. Each structural unit could be a single atom, a molecule or an ion. In C(s), the structural unit is a carbon atom. In I2(s), the structural unit is an I2 molecule. In NH4NO3(s), the structural units are NH4+ and NO3− ions. Unit cell the smallest building block that possesses the symmetry of the crystal lattice; a solid sample of any size can be “built” by stacking together unit cells. Cubic Packing Arrangements To investigate the cubic packing arrangements, we are going to imagine packing together identical, hard spheres each having a radius R. A. Simple cubic packing Let’s consider ”building” a simple cubic packing arrangement on top of a table. We generate the first layer by arranging the spheres as shown (on the left) below. Subsequent layers are added by lining up spheres with those in the previous layer as shown (on the right). Layer 3 Layer 1 Layer 2 Layer 1 Cubic Packing Arrangements A. Simple cubic packing (i) What is the relationship between the edge length of the unit cell (a) and the atom’s radius (R)? a R a a a = 2×R Cubic Packing Arrangements A. Simple cubic packing (ii) What is the number of spheres contained in one unit cell? a R a a Each cell contains eight one eighth of a sphere. Thus there are: 1 Number of spheres per unit cell 8 1 8 Cubic Packing Arrangements A. Simple cubic packing (iii) What is the coordination number of a sphere? a R a a Each sphere touches four spheres in the plane, one on top, and one underneath. Thus each sphere touches 6 other spheres. The coordination number equals 6. Cubic Packing Arrangements A. Simple cubic packing (iv) What is the fraction of empty space in a simple cubic crystal? a R a a The unit cell occupies a volume VUC = (a)3 = (2×R)3 = 8×R3. 4 3 The unit cell contains one sphere of volume: Vsphere R 3 The fraction of empty volume = (VUC – Vsphere)/VUC = 1 – /6 = 0.476 Cubic Packing Arrangements B. Body-centred cubic packing (i) What is the relationship between the edge length of the unit cell (a) and the atom’s radius (R)? a R Since the atoms are touching along the cube’s diagonal (body diagonal), its length equals: R + 2×R + R = 4×R Cubic Packing Arrangements B. Body-centred cubic packing (i) What is the relationship between the edge length of the unit cell (a) and the atom’s radius (R)? Body Diagonal = 4×R a What is the square diagonal? a a Cubic Packing Arrangements B. Body-centred cubic packing (i) What is the relationship between the edge length of the unit cell (a) and the atom’s radius (R)? Body Diagonal = 4×R a (Square Diagonal)2 = a2 + a2 = 2×a2 Square Diagonal 2a a 2 a a a Cubic Packing Arrangements B. Body-centred cubic packing (i) What is the relationship between the edge length of the unit cell (a) and the atom’s radius (R)? Body Diagonal = 4×R a (Square Diagonal)2 = a2 + a2 = 2×a2 Square Diagonal 2a 2 a (Body Diagonal)2 = a2 + (2×a)2 = 3×a2 Body Diagonal = 3×a = 4×R R 3 a 4 or a 4 R 3 a Cubic Packing Arrangements B. Body-centred cubic packing (ii) What is the number of spheres contained in one unit cell? a R Each corner is occupied by one eighth of an atom and there is a full atom at the center of the unit cell. Thus the cell contains: 1 Number of atoms per unit cell 1 8 1 1 2 8 Cubic Packing Arrangements B. Body-centred cubic packing (iii) What is the coordination number of a sphere? a R The atom at the center of the unit cell touches 8 other atoms. The coordination number equals 8. Cubic Packing Arrangements B. Body-centred cubic packing (iv) What is the fraction of empty space in a body centered cubic crystal? a R 3 64 3 4 3 R R The unit cell occupies a volume VUC = (a) = 3 3 3 4 3 3 The unit cell contains two spheres, each sphere of volume: Vsphere R The fraction of empty volume = (VUC – 2×Vsphere)/VUC = 1 3 0.319 8 Cubic Packing Arrangements C. Face-centred cubic packing (i) What is the relationship between the edge length of the unit cell (a) and the atom’s radius (R)? a R Cubic Packing Arrangements C. Face-centred cubic packing (i) What is the relationship between the edge length of the unit cell (a) and the atom’s radius (R)? (Square Diagonal)2 = a2 + a2 = 2×a2 a Square Diagonal = 2×a = 4×R a2 2R or R 1 2 2 a a a Cubic Packing Arrangements C. Face-centred cubic packing (ii) What is the number of spheres contained in one unit cell? a R Each one of the eight corners is occupied by one eighth of an atom and each one of the 6 faces is occupied by one half of an atom. 1 1 Number of atoms per unit cell 6 8 3 1 4 2 8 Cubic Packing Arrangements C. Face-centred cubic packing (iii) What is the coordination number of a sphere? The atom at the center of the face touches 4 atoms in the plane shown on the figure. Cubic Packing Arrangements C. Face-centred cubic packing (iii) What is the coordination number of a sphere? The atom at the center of the face touches 4 more atoms in the plane shown on the figure. Cubic Packing Arrangements C. Face-centred cubic packing (iii) What is the coordination number of a sphere? The atom at the center of the face touches 4 more atoms in the plane shown on the figure. Cubic Packing Arrangements C. Face-centred cubic packing (iii) What is the coordination number of a sphere? In total, the atom at the center of the face touches 12 atoms. The coordination number equals 12. Cubic Packing Arrangements C. Face-centred cubic packing (iv) What is the fraction of empty space in a face-centered cubic crystal? a R The unit cell occupies a volume VUC = (a)3 = 2 3 2 R 16 2 R 3 4 3 0.260 The fraction of empty volume = (VUC – 4×Vsphere)/VUC = 1 3 2 3 The unit cell contains four spheres, each sphere of volume: Vsphere R Intermolecular Forces: Liquids and Solids ● Phases and Phase Diagrams ● Liquids and Liquid Properties ● Intermolecular Forces ● Heating Curves ● Introduction to Solids ● Cubic Packing Arrangements ● Closest-Packed Structures ● Density of a Crystalline Solid ● Ionic Solids and Interstitial Sites ● The Born-Haber Cycle Closest Packed Structures Same layer as the layer with the grey balls. Different layer from the layer with the grey balls. Closest Packed Structures “x” type dimple (vertically aligned with a sphere in layer 1) “y” type dimple (not vertically aligned with a sphere in layer 1 or layer 2) There are two types of “dimples” to choose from when forming layer 3!! Closest Packed Structures “x” type dimple (vertically aligned with a sphere in layer 1) choose “x” only “y” type dimple (not vertically aligned with a sphere in layer 1 or layer 2) choose “y” only Spheres in layer 3 are vertically aligned with those in layer 1. That is, layer 3 is a repeat of layer 1. Spheres in layer 3 are not aligned vertically with those in layer 1 or layer 2. Layer 3 is a distinct layer. ABAB ... closest-packing ABCABC ... closest-packing Closest Packed Structures Spheres in layer 3 are vertically aligned with those in layer 1. That is, layer 3 is a repeat of layer 1. Spheres in layer 3 are not aligned vertically with those in layer 1 or layer 2. Layer 3 is a distinct layer. ABAB ... closest-packing ABCABC ... closest-packing The unit cell for ABAB ... closest-packing is “hexagonal”. Thus, this type of closest-packing is also called “hexagonal closest-packing” or hcp for short. The unit cell for ABCABC ... closestpacking is “face-centred cubic”. Thus, this type of closest-packing is also called “cubic closest-packing” or ccp for short. Closest Packed Structures The unit cell for ABAB ... closest-packing is “hexagonal”. Thus, this type of closest-packing is also called “hexagonal closest-packing” or hcp for short. The unit cell for ABCABC ... closestpacking is “face-centred cubic”. Thus, this type of closest-packing is also called “cubic closest-packing” or ccp for short. You are expected to remember that cubic closest-packing (ccp) and face-centered cubic (fcc) are the same!! Intermolecular Forces: Liquids and Solids ● Phases and Phase Diagrams ● Liquids and Liquid Properties ● Intermolecular Forces ● Heating Curves ● Introduction to Solids ● Cubic Packing Arrangements ● Closest-Packed Structures ● Density of a Crystalline Solid ● Ionic Solids and Interstitial Sites ● The Born-Haber Cycle Density of a Crystalline Solid The density of a solid depends on the microscopic details of the packing arrangement. d m mcell V Vcell M natoms atom NA a3 mass of one atom Matom is molar mass, in g mol−1 NA = 6.022×1023 mol −1 Keep in mind: The formula d = ncell Matom /(NA a3) should be used only for identical hard spheres in a cubic lattice. If you have more than one type of sphere (e.g. of different sizes or masses) or unit cell that is not cubic, then you should start from d = mcell / Vcell. Density of a Crystalline Solid Example: Tungsten, W, crystallizes in one of the three cubic arrangements. If the edge length of the unit cell is 321 pm and the density is 18.5 g cm-3, then what type of crystal lattice does tungsten have? What is the radius of a tungsten atom? m mcell d V Vcell M atom natoms NA a3 Matom = 184 g.mol1 Density of a Crystalline Solid Example: Tungsten, W, crystallizes in one of the three cubic arrangements. If the edge length of the unit cell is 321 pm and the density is 18.5 g cm-3, then what type of crystal lattice does tungsten have? What is the radius of a tungsten atom? m mcell d V Vcell M atom natoms NA a3 Matom = 184 g.mol1 d a3 natoms M atom NA 18.5 ( g.cm 3 ) (32110 10 cm)3 natoms 2.003 2 1 184 g.mol 23 1 6.022 10 mol Density of a Crystalline Solid Example: Tungsten, W, crystallizes in one of the three cubic arrangements. If the edge length of the unit cell is 321 pm and the density is 18.5 g cm-3, then what type of crystal lattice does tungsten have? What is the radius of a tungsten atom? Relationship # of atoms/unit cell between a and R Simple cubic Body-centered cubic R = a/2 1 R 2 R Face-centered cubic 4 3 a 4 3 321 139 pm 4 R 1 2 2 a Density of a Crystalline Solid The edge length can be determined experimentally using x-ray diffraction. When x-rays are passed through a crystalline solid, the x-rays are deflected from their paths by the atoms of the solid and interfere with each other to produce an interference pattern – a “diffraction pattern” – that can be analyzed to determine the geometry of the crystal lattice. Relationship # of atoms/unit cell between a and R Simple cubic 1 Body-centered cubic 2 Face-centered cubic 4 R = a/2 R R 3 a 4 1 2 2 a REVIEW Introduction to Solids For crystalline solids, we use the following concepts to characterize the pattern of the packing arrangement. Crystal lattice three dimensional array of points that shows how the structural units are arranged in space. Each structural unit could be a single atom, a molecule or an ion. In C(s), the structural unit is a carbon atom. In I2(s), the structural unit is an I2 molecule. In NH4NO3(s), the structural units are NH4+ and NO3− ions. Unit cell the smallest building block that possesses the symmetry of the crystal lattice; a solid sample of any size can be “built” by stacking together unit cells. REVIEW Introduction to Solids When we focus on the geometry of the packing arrangements, we find that there are seven basic shapes for unit cells. The shape of each unit cell is described in terms of three lengths (a, b and c) and three angles (, , and g). We will focus primarily on cubic unit cells. The others are mentioned only to emphasize that there are other types/shapes of unit cells besides cubic unit cells. cubic a=b=c = = g = 90o trigonal a=b=c tetragonal = = g ≠ 90o a=b≠c hexagonal = = g = 90o a=b≠c o; = = 90 monoclinic and g = 120o a≠b≠c = g = 90o triclinic and ≠ 90o a≠b≠c orthorhombic ≠ ≠ g ≠ 90o g a≠b≠c = = g = 90o a b c REVIEW Introduction to Solids When we focus on the geometry of the packing arrangements, we find that there are seven basic shapes for unit cells. The shape of each unit cell is described in terms of three lengths (a, b and c) and three angles (, , and g). We will focus primarily on cubic unit cells. The others are mentioned only to emphasize that there are other types/shapes of unit cells besides cubic unit cells. cubic a=b=c = = g = 90o trigonal a=b=c tetragonal = = g ≠ 90o a=b≠c hexagonal = = g = 90o a=b≠c o monoclinic = = 90 ;o and g = 120 a≠b≠c = g = 90o triclinic and ≠ 90o a≠b≠c orthorhombic ≠ ≠ g ≠ 90o a≠b≠c = = g = 90o a a a REVIEW Density of a Crystalline Solid The edge length can be determined experimentally using x-ray diffraction. When x-rays are passed through a crystalline solid, the x-rays are deflected from their paths by the atoms of the solid and interfere with each other to produce an interference pattern – a “diffraction pattern” – that can be analyzed to determine the geometry of the crystal lattice. Relationship # of atoms/unit cell between a and R Simple cubic 1 Body-centered cubic 2 Face-centered cubic 4 R = a/2 R = 0.50×a 3 a 4 R = 0.43×a R R 1 2 2 a R = 0.35×a Intermolecular Forces: Liquids and Solids ● Phases and Phase Diagrams ● Liquids and Liquid Properties ● Intermolecular Forces ● Heating Curves ● Introduction to Solids ● Cubic Packing Arrangements ● Closest-Packed Structures ● Density of a Crystalline Solid ● Ionic Solids and Interstitial Sites ● The Born-Haber Cycle Ionic Solids and Interstitial Sites Up to this point, we’ve focused on packing together identical hard spheres. Now, we’ll extend that model so that we can describe the structures of binary ionic solids. For many ionic solids, it is often the case that one of the ions forms a cubic lattice and the other ion occupies “holes” in that lattice. In order to understand the structures of ionic crystals, we must first examine the types of holes we can have. “Holes” (or interstitial sites) are named according to the “coordination number” of a small sphere that just fits into that hole. The types of holes that interest us the most are cubic, tetrahedral and octahedral holes. Ionic Solids and Interstitial Sites trigonal hole The small sphere has a coordination number of three. Ionic Solids and Interstitial Sites tetrahedral hole coordination # = 4 Ionic Solids and Interstitial Sites octahedral hole coordination # = 6 top view side view Ionic Solids and Interstitial Sites cubic hole coordination # = 8 Ionic Solids and Interstitial Sites Example: Within the face-centered cubic (fcc) unit cell, there are both octahedral and tetrahedral holes. Examine the fcc unit cell to identify the locations of the octahedral and tetrahedral holes. How many holes of each type are there per unit cell? One octahedral hole ¼ of an octahedral hole Ionic Solids and Interstitial Sites Example: Within the face-centered cubic (fcc) unit cell, there are both octahedral and tetrahedral holes. Examine the fcc unit cell to identify the locations of the octahedral and tetrahedral holes. How many holes of each type are there per unit cell? One octahedral hole ¼ of an octahedral hole There is ¼ of an octahedral hole (per edge) and an octahedral hole at the centre of the cell. # octahedral holes = ¼ hole/edge × 12 edges + 1 = 4 (per fcc unit cell) For the fcc cell, there are 4 spheres per cell and 4 octahedral holes holes. The ratio of spheres-to-holes is: # spheres : # octahedral holes = 4 : 4 = 1 : 1 Ionic Solids and Interstitial Sites Example: Within the face-centered cubic (fcc) unit cell, there are both octahedral and tetrahedral holes. Examine the fcc unit cell to identify the locations of the octahedral and tetrahedral holes. How many holes of each type are there per unit cell? b a tetrahedral hole c d There is one tetrahedral hole associated with each corner of the fcc cell (that is, the sphere at each corner can be considered to be the “cap” of a tetrahedron). Thus, # tetrahedral holes = 8 (per fcc cell) For the fcc cell, there are 4 spheres per celland 8 tetrahedral holes. The ratios of spheres-to-holes are: # spheres : # tetrahedral holes = 4 : 8 = 1 : 2 Ionic Solids and Interstitial Sites Example: Within the face-centered cubic (fcc) unit cell, there are both octahedral and tetrahedral holes. Examine the fcc unit cell to identify the locations of the octahedral and tetrahedral holes. How many holes of each type are there per unit cell? b a tetrahedral hole c d In general: In a closest-packed structure containing N spheres, where N is a very large number, there are N octahedral holes and 2N tetrahedral holes. Ionic Solids and Interstitial Sites How big are tetrahedral, octahedral and cubic holes? Octahedral holes. Let ro be the radius of a sphere that just fits into the octahedral hole. Let ro be the radius of a sphere that just fits into the octahedral hole. (2 R) 2 (2 R) 2 ( R 2ro R) 2 2ro 2R 8 R 2 4 ( R ro ) 2 2 R 2 ( R ro ) 2 2 R R ro 2R ro R (1 2 ) 0.414 R Ionic Solids and Interstitial Sites How big are tetrahedral, octahedral and cubic holes? Cubic holes: Let ro be the radius of a sphere that just fits into the cubic hole. Let rc be the radius of a sphere that just fits into the cubic hole. This sphere has its centre at the midpoint of the bodydiagonal. Therefore, we need to find the length of the body-diagonal. 2R BD R 2ro R 2 ( R ro ) Ionic Solids and Interstitial Sites How big are tetrahedral, octahedral and cubic holes? Cubic holes: Let ro be the radius of a sphere that just fits into the cubic hole. BD R 2ro R 2 ( R ro ) Diagonal2 = a2 a2 2 a2 Diagonal = a 2a a 2 a Ionic Solids and Interstitial Sites How big are tetrahedral, octahedral and cubic holes? Cubic holes: Let ro be the radius of a sphere that just fits into the cubic hole. BD R 2ro R 2 ( R ro ) BD 2 ( 2a) 2 a 2 2 a 2 a 2 3 a 2 BD 3 a 3 (2 R) 2 3R BD 2 ( R ro ) 2 3R 2a ro ( 3 1) R 0.732 R a Ionic Solids and Interstitial Sites How big are tetrahedral, octahedral and cubic holes? Tetrahedral holes: Let ro be the radius of a sphere that just fits into the tetrahedral hole. b a b a c 2×ro d c d Ionic Solids and Interstitial Sites How big are tetrahedral, octahedral and cubic holes? Tetrahedral holes: Let ro be the radius of a sphere that just fits into the tetrahedral hole. b a b a x? c 2×ro d c BD R 2ro R 2 ( R ro ) d Ionic Solids and Interstitial Sites How big are tetrahedral, octahedral and cubic holes? Tetrahedral holes: Let ro be the radius of a sphere that just fits into the tetrahedral hole. b a b 2×R a x d c d x c x 2 x 2 2 x 2 ( 2 R) 2 x 2R Ionic Solids and Interstitial Sites How big are tetrahedral, octahedral and cubic holes? Tetrahedral holes: Let ro be the radius of a sphere that just fits into the tetrahedral hole. b a b a x= c 2×ro d 2R d c BD R 2ro R 2 ( R ro ) BD 2 4 ( R ro )2 (2R)2 ( 2R) 2 6 R 2 BD 2 ( R ro ) 6 R Ionic Solids and Interstitial Sites How big are tetrahedral, octahedral and cubic holes? Tetrahedral holes: Let ro be the radius of a sphere that just fits into the tetrahedral hole. b a b a x= c 2×ro d c 6 ro ( 1) R 0.225 R 2 2R d Ionic Solids and Interstitial Sites Summary of hole types and the radius ratio rules Trigonal Tetrahedral Octahedral cubic Holes Trigonal Tetrahedral Octahedral cubic Relationship Too small to worry about 0.225 R 0.414 R 0.732 R Ionic Solids and Interstitial Sites Radius Ratio Rules for Ionic Solids Let R+ be the radius of the positive ion in a binary ionic solid and let R− be the radius of the negative ion. Positive ions occupy tetrahedral sites in the R lattice of negative ions. The coordination 0.225 0.414 R number of each positive ion is 4. Note: The positive ions are too big for the tetrahedral sites and thus the negative ions are forced apart a little this increases the attraction between the “+” and “−” ions and decreases the repulsion between the “−” ions. 0.414 R 0.732 R 0.732 R R Positive ions occupy octahedral sites in the lattice of negative ions. The coordination number of each positive ion is 6. Positive ions occupy cubic sites in the lattice of negative ions. The coordination number of each positive ion is 8. Note: The rules above are just guidelines. There are many exceptions. Ionic Solids and Interstitial Sites Sodium Chloride Structure (also called “rock salt structure”) Cl− ions form a face-centered cubic (fcc) lattice and Na+ ions occupy 100% of the octahedral holes in the chloride lattice. Na Cl Ionic Solids and Interstitial Sites Cesium Chloride Structure Cl− ions form a simple cubic lattice and Cs+ ions occupy cubic holes in the chloride lattice. Cs Cl Ionic Solids and Interstitial Sites Zinc Blende Structure S2− ions form a face-centered cubic (fcc) lattice and Zn2+ ions occupy half (50%) of the tetrahedral hole. Why are only half of the tetrahedral holes occupied? How many spheres: 4 S2 How many tetrahedral holes: 8 for only 4 Zn2+ Ionic Solids and Interstitial Sites Zinc Blende Structure S2− ions form a face-centered cubic (fcc) lattice and Zn2+ ions occupy half (50%) of the tetrahedral hole. Why are only half of the tetrahedral holes occupied? There are 4 S2− ions per cell. There must be four Zn2+ ions per cell because ZnS is a 1:1 salt. Therefore, only 4 of 8 tetrahedral holes are occupied by Zn2+ ions. REVIEW Introduction to Solids For crystalline solids, we use the following concepts to characterize the pattern of the packing arrangement. Crystal lattice three dimensional array of points that shows how the structural units are arranged in space. Each structural unit could be a single atom, a molecule or an ion. In C(s), the structural unit is a carbon atom. In I2(s), the structural unit is an I2 molecule. In NH4NO3(s), the structural units are NH4+ and NO3− ions. Unit cell the smallest building block that possesses the symmetry of the crystal lattice; a solid sample of any size can be “built” by stacking together unit cells. REVIEW Introduction to Solids When we focus on the geometry of the packing arrangements, we find that there are seven basic shapes for unit cells. The shape of each unit cell is described in terms of three lengths (a, b and c) and three angles (, , and g). We will focus primarily on cubic unit cells. The others are mentioned only to emphasize that there are other types/shapes of unit cells besides cubic unit cells. cubic a=b=c = = g = 90o trigonal a=b=c tetragonal = = g ≠ 90o a=b≠c hexagonal = = g = 90o a=b≠c o; = = 90 monoclinic and g = 120o a≠b≠c = g = 90o triclinic and ≠ 90o a≠b≠c orthorhombic ≠ ≠ g ≠ 90o g a≠b≠c = = g = 90o a b c REVIEW Introduction to Solids When we focus on the geometry of the packing arrangements, we find that there are seven basic shapes for unit cells. The shape of each unit cell is described in terms of three lengths (a, b and c) and three angles (, , and g). We will focus primarily on cubic unit cells. The others are mentioned only to emphasize that there are other types/shapes of unit cells besides cubic unit cells. cubic a=b=c = = g = 90o trigonal a=b=c tetragonal = = g ≠ 90o a=b≠c hexagonal = = g = 90o a=b≠c o monoclinic = = 90 ;o and g = 120 a≠b≠c = g = 90o triclinic and ≠ 90o a≠b≠c orthorhombic ≠ ≠ g ≠ 90o a≠b≠c = = g = 90o a a a REVIEW Density of a Crystalline Solid The edge length can be determined experimentally using x-ray diffraction. When x-rays are passed through a crystalline solid, the x-rays are deflected from their paths by the atoms of the solid and interfere with each other to produce an interference pattern – a “diffraction pattern” – that can be analyzed to determine the geometry of the crystal lattice. Relationship # of atoms/unit cell between a and R Simple cubic 1 Body-centered cubic 2 Face-centered cubic 4 R = a/2 R = 0.50×a 3 a 4 R = 0.43×a R R 1 2 2 a R = 0.35×a REVIEW Ionic Solids and Interstitial Sites Summary of hole types and the radius ratio rules Trigonal Tetrahedral Octahedral cubic Holes Trigonal Tetrahedral Octahedral cubic Relationship Too small to worry about 0.225 R 0.414 R 0.732 R Ionic Solids and Interstitial Sites Fluorite Structure Ca2+ ions form a fcc lattice and F− ions occupy all of the tetrahedral holes. Thus, there are 4 Ca2+ ions per unit cell and 8 F− ions. How many spheres: 4 Ca2+ How many tetrahedral holes: 8 F Ionic Solids and Interstitial Sites Antifluorite Structure O2 Na Sodium oxide, Na2O, adopts the so-called antifluorite structure. The antifluorite structure is the “reverse” of the fluorite structure in the sense that the negative ions form a fcc lattice and the positive ions occupy all of the tetrahedral holes. In the case of Na2O, the O2− ions form a fcc lattice and the Na+ ions occupy 100% of the tetrahedral holes. Ionic Solids and Interstitial Sites Example: In beryllium oxide, BeO, the oxide ions form a face-centred cubic lattice and the beryllium ions occupy tetrahedral sites in the lattice of O2- ions. What fraction of the holes is occupied by the Be2+ ions? (Ans: 50%) How many spheres: 4 O2 How many tetrahedral holes: 8 tetrahedral holes for Be2 With 8 negative charges from O2 anions and 16 positive charges from Be2+ cations, the charge balance would not work. Only 4 Be2+ generating 8 positive charges can be present. 50% of the tetrahedral holes can be occupied by Be2+ cations. Ionic Solids and Interstitial Sites Example: To “build” the sodium chloride structure, we would first arrange Cl ions in a face-centred cubic structure and then insert Na+ ions into the octahedral sites of the Cl lattice. Assume that Na+ and Cl are hard spheres with radii of 97 pm and 181 pm, respectively. Calculate the density of sodium chloride, in g cm3. (Ans: 2.26 g cm−3) a = 2×(97+181) = 556 pm Na 4 spheres (Cl) 4 octahedral holes (Na+) Cl a d 4 ( M Na M Cl ) 4 (23 35.5 g / mol ) 3 2 . 26 g . cm a3 N A (556 10 10 cm) 3 (6.022 10 23 mol 1 ) Ionic Solids and Interstitial Sites Example: In cesium chloride, the Cl ions form a simple cubic lattice and the Cs+ ions occupy cubic holes in the chloride lattice. However, the Cs+ ions force the Cl ions apart so that none of the chloride ions are in direct contact with each other. If the density of cesium chloride, CsCl, is 3.988 g cm3, then what is the distance (R+ + R) ? (Ans: 357 pm) 1 spheres (Cl) 1 cubic hole (Cs+) d Cs a Cl a3 ( M Cs M Cl ) a3 N A a3 ( M Cs M Cl ) d NA where BD 2 ( R R ) 3 a M Cs M Cl 132.9 35.5 8 3 4 . 12 10 cm 412 pm 3 23 d NA 3.988 g.cm 6.022 10 Ionic Solids and Interstitial Sites Example: In cesium chloride, the Cl ions form a simple cubic lattice and the Cs+ ions occupy cubic holes in the chloride lattice. However, the Cs+ ions force the Cl ions apart so that none of the chloride ions are in direct contact with each other. If the density of cesium chloride, CsCl, is 3.988 g cm3, then what is the distance (R+ + R) ? (Ans: 357 pm) a 412 pm BD 3 a 3 412 714 pm a Cs Cl BD 714 pm 2 ( R R ) R R 714 pm 357 pm 2 Intermolecular Forces: Liquids and Solids ● Phases and Phase Diagrams ● Liquids and Liquid Properties ● Intermolecular Forces ● Heating Curves ● Introduction to Solids ● Cubic Packing Arrangements ● Closest-Packed Structures ● Density of a Crystalline Solid ● Ionic Solids and Interstitial Sites ● The Born-Haber Cycle The Born Haber Cycle The stability of an ionic solid is quantified in terms of its lattice energy. Lattice Energy = energy change when gas phase ions combine to form an ionic solid Lattice energy (Hlattice) for NaCl(s) Hlattice Na+(g) + Cl−(g) → NaCl(s) The lattice energy cannot be measured directly. However, we can obtain it indirectly from other thermochemical data using the Born-Haber cycle. The Born-Haber cycle involves the following steps: • Write down the “formation reaction” for the solid from the elements under standard conditions (T = 25 oC and P = 105 bar). • Convert the elements into gas-phase atoms. • Convert the atoms into gas-phase ions. • Combine the ions to form the solid. The Born Haber Cycle Born-Haber cycle for NaCl(s) • Write down the “formation reaction” for the solid. • Convert the elements into gas-phase atoms. • Convert the atoms into gas-phase ions. • Combine the ions to form the solid. Hfo Na(s) + ½ Cl2(g) NaCl(s) Hsubo (sublimation) Na(g) + IE(1) (first ionization energy) Na+(g) + ½ DCl-Cl (dissociation) Cl(g) EA(1) (first electron affinity) Cl(g) Hlatticeo The Born Haber Cycle Born-Haber cycle for NaCl(s) State function does not depend on the path: University of d(home – UW) = constant path(UW home) varies The Born Haber Cycle Born-Haber cycle for NaCl(s) • Write down the “formation reaction” for the solid. • Convert the elements into gas-phase atoms. • Convert the atoms into gas-phase ions. • Combine the ions to form the solid. Hfo Na(s) + ½ Cl2(g) NaCl(s) Hsubo (sublimation) Na(g) + IE(1) (first ionization energy) Na+(g) + ½ DCl-Cl (dissociation) Cl(g) EA(1) (first electron affinity) Hlatticeo Cl(g) Apply Hess’ Law: Hfo = Hsubo + IE(1)Na(g) + ½ DCl-Cl + EA(1)Cl(g) + Hlattice Thus: Hlattice = (Hsubo + IE(1)Na(g) + ½ DCl-Cl + EA(1)Cl(g)) Hfo The Born Haber Cycle Born-Haber cycle for MgF2(s) • Write down the “formation reaction” for the solid. • Convert the elements into gas-phase atoms. • Convert the atoms into gas-phase ions. • Combine the ions to form the solid. Hfo Mg(s) + F2(g) MgF2(s) Hsubo (sublimation) Mg(g) + IE(1) + IE(2) (first and second ionization energy) DF-F (dissociation) 2 F(g) EA(1) (first electron affinity) Hlatticeo Mg2+(g) + 2 F(g) Apply Hess’ Law: Hfo = Hsubo + IE(1)Mg(g) +IE(2)Mg(g) + DF-F + 2×EA(1)F(g) + Hlattice Thus: Hlattice = (Hsubo + IE(1)Mg(g) +IE(2)Mg(g) + DF-F + 2×EA(1)F(g)) + Hfo The Born Haber Cycle Born-Haber cycle for NaCl(s) Hess’ Law: Hfo = Hsubo + IE(1)Na(g) + ½ DCl-Cl + EA(1)Cl(g) + Hlattice Born-Haber cycle for MgF2(s) Hess’ Law: Hfo = Hsubo + IE(1)Mg(g) +IE(2)Mg(g) + DF-F + 2×EA(1)F(g) + Hlattice By comparing the expressions we wrote for NaCl(s) and MgCl2(s), we can see that the expression we obtain for ΔHfo will vary from salt to salt. Therefore, to obtain the correct expression, you must first construct the Born-Haber cycle. Intermolecular Forces: Liquids and Solids ● Phases and Phase Diagrams ● Liquids and Liquid Properties ● Intermolecular Forces ● Heating Curves ● Introduction to Solids ● Cubic Packing Arrangements ● Closest-Packed Structures ● Density of a Crystalline Solid ● Ionic Solids and Interstitial Sites ● The Born-Haber Cycle