Lecture 3-22-11
advertisement
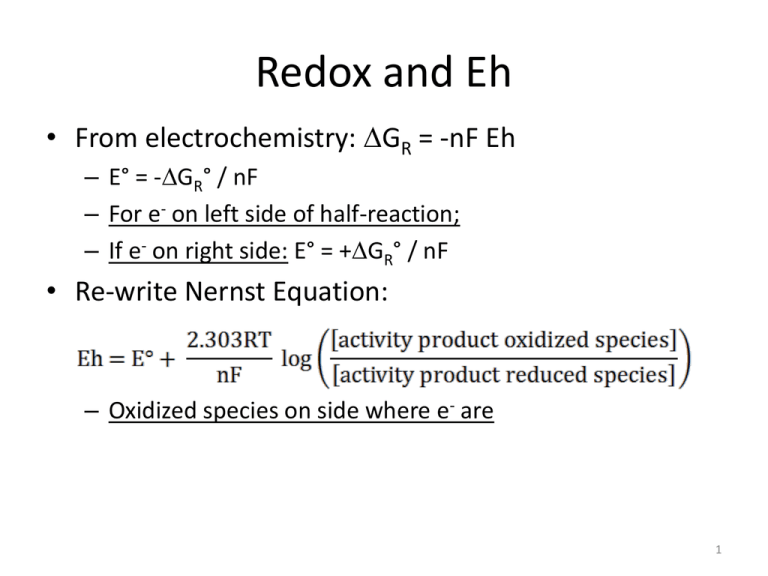
Redox and Eh • From electrochemistry: GR = -nF Eh – E° = -GR° / nF – For e- on left side of half-reaction; – If e- on right side: E° = +GR° / nF • Re-write Nernst Equation: – Oxidized species on side where e- are 1 Measuring Eh • The Eh value is usually not very accurate in natural waters because of a lack of redox equilibrium – One half of redox pair often below detection • Best to use Eh as a semi-quantitative measurement, giving you a relative idea of the redox potential of the water 2 Eh – pH Diagrams • A different type of stability diagram, but using Eh as variable instead of activity – Lines indicate equilibrium – Domains define areas of stability for minerals and aqueous species 3 O2 and H2 are present in entire H2O stability range Oxidizing and reducing with respect to SHE Oxidizing environments may contain only small amounts of O2 4 1 We determine what species, minerals are in diagram +++ Fe ++ Diagram Fe , T = 25 °C , P = 1.013 bars, a [main] = 10 , a [H2 O] = 1, a [SO 4 ] = 10 –5 (speciates) Eh (volts) .5 -- Hematite Fe –6 0 Pyrite –.5 Troilite 0 2 4 6 8 pH 10 12 ++ Magnetite FeO(c) 25°C 14 Walt Fri May 05 2006 5 Evolution of Water Chemistry 6 Source of dissolved species • Primarily from chemical weathering • Primary minerals + acid secondary minerals + dissolved ions – The essential ingredients needed for chemical weathering are water and acid 7 Precipitation • Soil water and groundwater start out as precipitation – Very dilute (low TDS), in equilibrium with atmospheric gases (O2, CO2, N2) • Precipitation passes through the soil zone and unsaturated zone 8 Soils and Weathering • In most areas, soils are the first geologic unit to come into contact with precipitation – If soil has organic matter, OM decays, consuming O2 and producing CO2 • CH2O + O2(g) → CO2(g) + H2O • CO2 + H2O H2CO3 HCO3- + H+ • Soil PCO2 = 10-3 – 10-1 atm – (atmosphere = 10-3.5) – Due to production of acid (CO2) soils have the highest rate of chemical weathering – TDS increases as minerals dissolve, ions desorbed 9 Unsaturated Zone • After passing through the soil zone, water percolates down through the unsaturated zone – Thickness of unsaturated zone is primarily a function of annual precipitation (climate) • Also affected by lithology, topography, plant species, nearness to surface water • Water can move through the unsaturated zone quickly, or can remain for a long time (years) – Dissolution/precipitation reactions can occur in the unsaturated zone, altering water chemistry 10 Groundwater Chemistry Evolution • By the time water reaches the water table, it has acquired the chemical signature of the geologic materials it is flowing through • As it moves along a groundwater flow path, the chemistry continues to evolve • Evolutionary sequence controlled by mineral availability and solubility – High availability: carbonates and felsic minerals – High solubility: gypsum/anhydrite, evaporites 11 Evolution of Groundwater Chemistry 12 Open vs. closed systems • Soil and shallow groundwater (< 10 ft below water table) are open systems with respect to gases (CO2 and O2) – Gaseous exchange with the atmosphere (or soil gas), which is at or near equilibrium saturation – As CO2 and O2 are consumed, replaced by CO2 from atmosphere – As CO2 is generated, it will degas • Deeper groundwater is a closed system with respect to gases – Water is isolated from the atmosphere – If gases are consumed, their concentrations decrease; if generated, concentrations increase 13 General trends in groundwater with increasing age and/or depth • O2: rapidly consumed by biological activity (oxidation of organic matter or reduced minerals) • pH: usually rises along a flow path as H+ is consumed during weathering reactions – A closed system has finite acidity – pH can fall by oxidation of sulfide minerals • HCO3-: concentration increases because H+ in H2CO3 consumed 14 Trends with age/depth • As groundwater migrates, concentration of TDS and most major ions increases • Anions – Chebotarev took 10,000 groundwater samples from large sedimentary basins in Australia and determined that groundwater evolves towards seawater composition – Determined that relative abundances of anions changed with travel distance/age • HCO3- HCO3- + SO42- SO42- + HCO3- SO42- + Cl- Cl- + SO42- Cl15 Groundwater Anion Evolution SO4 0 Tri-linear Diagram: Used in Piper Diagrams 100 20 80 40 60 60 40 80 20 Young Very Old 100 0 HCO3 + CO3 20 40 60 80 0 100 Cl 16 Trends with age/depth • Cations – More difficult to generalize trends – Most common trend: Ca2+, then Ca-Na, Na-Ca, finally Na+ – Driven by cation exchange and CaCO3 precipitation • Redox Species – Sequential reduction of oxidized species 17 Trends with age/depth • Groundwater Chemistry Zones – Upper: active groundwater circulation, relatively weathered (leached) rocks, Ca2+ - HCO3- dominate, low TDS • Usually not a lot of soluble minerals (like halite and gypsum) • HCO3- dominant anion, Ca2+ commonly dominant cation, relatively low TDS (< 500 mg/L) 18 Trends with age/depth • Groundwater Chemistry Zones – Intermediate: less active flow, unweathered rocks, SO42- dominant anion, Na+ increases but Ca2+usually still important, higher TDS – Lower: slow circulation, unweathered rocks, Na+ Cl- dominant ions, high TDS • Highly soluble minerals common 19 Evolution of Groundwater Chemistry Low TDS Intermediate TDS Aquitard: TDS high relative to aquifers High TDS 20 Mineralogy and Water Chemistry • Identity of rocks and minerals along groundwater flowpath an important variable affecting water chemistry 21 Mineralogy of Igneous Rocks: Bowen’s Reaction Series At/Near Earth’s Surface: Less Stable Everything else being equal, Ca > Na > K More Stable 22 Mineralogy of Igneous Rocks: Bowen’s Reaction Series Mafics Felsics 23 Igneous Rock Type and Water Chemistry • Mafic igneous rocks – High TDS, high Si – Mg2+ and Ca2+ dominant cations – Anions: HCO3- • Felsic igneous and metamorphic rocks – Relatively low (< 500 mg/L) TDS – Anions: HCO3- dominant, F- can be characteristic – Cations: Ca2+ and Na+ dominant • Fine-grained or glassy rocks – High TDS because of high mineral surface area or no mineral structure 24 Sedimentary Rock Type and Water Chemistry • Sandstone – Variable, dependent on mineral composition and how “pure” sandstone is – Most often like felsics, but higher TDS • Limestone/dolomite – – – – – TDS > igneous Cations: Ca and Mg, little Na Anions: HCO3Si varies Dolomite: Ca and Mg equimolar 25 Sedimentary Rock Type and Water Chemistry • Shale – Main minerals quartz and illite are relatively unreactive – Long contact time can lead to high TDS – Most shales form in marine environments, and Na+ and Cl- can be elevated from original porewater – SO42- if pyrite is present, and from porewater – Plenty of Si 26 Atmospheric Solids and Water Chemistry • Atmospheric input (dust, etc.) – Can provide significant amounts of weatherable material in all climates – In arid regions, this can be a dominant source – Laterites on limestone in Bahamas and Amazon: Al and Fe from dust 27 Chemical Weathering: Climate and Topography • Climate – As precipitation increases, mineral dissolution increases, more acid to attack the minerals – For constant precipitation, weathering rate increases with temperature • Topography – Some debate about this, but the majority of evidence indicates decreased chemical weathering with increasing elevation – Probably related to thinner soils, cooler temperatures 28 Water Chemistry: Information on Weathering Reactions • Knowing starting and ending solution chemistry of a system, we can infer what reactions have taken place to produce the ending solution – Reaction-Path Modeling – In addition to water chemistry, need information on minerals present – As groundwater migrates along a flow path, reactions occur: • Dissolution adds ions • Mineral precipitation removes ions – The change in water chemistry = the sum of all dissolution/precipitation reactions 29 Water Chemistry: Information on Weathering Reactions • Garrels and Mackenzie (1967) first to develop reaction path modeling concept – Applied on watershed scale (Sierra Nevadas) – Initial solution was precipitation (rainfall and snowmelt) – Ending solution was spring chemistry 30 Example: granitic springs in Sierra Nevadas • Information that helped characterize the system: • Geology: Rocks classified as quartz diorite and quartz microcline gneiss • Primary minerals – Feldspars: albite (Na), microcline (K), anorthite (Ca) • Average feldspar: andesine (Ca and Na) – Quartz – Biotite/hornblende • Climate: high elevation (2-3 km), cool T, high winter snowfall, summer thunderstorms 31 Example: granitic springs in Sierra Nevadas • Start building conceptual model: • As precipitation recharges the subsurface, which primary minerals would weather most readily? Least readily? 32 Mineralogy of Igneous Rocks: Bowen’s Reaction Series At/Near Earth’s Surface: Less Stable More Stable 33 Example: granitic springs in Sierra Nevadas • G&M predict decreasing weatherability: Caplagioclase Na-plagioclase Biotite/hornblende K feldspar quartz • What are expected secondary minerals? – Clays: kaolinite and smectite – Amorphous SiO2 – CaCO3? 34 Example: granitic springs in Sierra Nevadas • Ending solutions: Ephemeral and perennial springs – Ephemeral: short residence time (up to several years), low TDS and pH – Perennial: higher residence time (10-100’s yrs), higher TDS and pH • Reaction path model – Starting point: snow chemistry – Ending point: spring chemistry – Difference between the two result of reactions involving dissolution of primary minerals, precipitation of secondary minerals 35 Ephemeral springs in Sierra Nevadas • Began by subtracting snow water chemistry from spring water chemistry to determine how much of each ion/species added SiO2 Ca Mg Na K HCO3 SO4 Cl ephemeral mM 0.273 0.078 0.029 0.134 0.028 0.328 0.01 0.014 - snow water mM 0.270 0.068 0.022 0.110 0.020 0.310 0 0 36 Ephemeral springs in Sierra Nevadas • All SO4 and Cl removed; none added in the subsurface • Remaining species added by reactions SiO2 Ca Mg Na K HCO3 SO4 Cl ephemeral mM 0.273 0.078 0.029 0.134 0.028 0.328 0.01 0.014 - snow water mM 0.270 0.068 0.022 0.110 0.020 0.310 0 0 37 Ephemeral springs in Sierra Nevadas • Hypothesis: plagioclase, biotite and K-feldspar each weathers to kaolinite, amorphous SiO2, and dissolved ions – Allow spring water to back-react with kaolinite to see if could get original minerals – First, react Na, Ca, HCO3, and SiO2 with kaolinite to make plagioclase • All Na and Ca used up • Resulting plagioclase composition close to what is found 38 Ephemeral springs in Sierra Nevadas SiO2 Ca Mg Na K HCO3 SO4 Cl ephemeral - snow water -plagioclase mM mM mM 0.273 0.270 0.050 0.078 0.068 0 0.029 0.022 0.022 0.134 0.110 0 0.028 0.020 0.020 0.328 0.310 0.064 0.01 0 0 0.014 0 0 • Next, react all Mg along with K, HCO3, and SiO2 to make biotite (KMg3AlSi3O10(OH)2) 39 Ephemeral springs in Sierra Nevadas SiO2 Ca Mg Na K HCO3 SO4 Cl ephemeral - snow water -plagioclase mM mM mM 0.273 0.270 0.050 0.078 0.068 0 0.029 0.022 0.022 0.134 0.110 0 0.028 0.020 0.020 0.328 0.310 0.064 0.01 0 0 0.014 0 0 -biotite mM 0.035 0 0 0 0.013 0.013 0 0 • Remaining K, HCO3, and SiO2 used to form K-feldspar • 4% of original SiO2 remains, good enough 40 Ephemeral springs in Sierra Nevadas • Resulting balance worked remarkably well, explaining the concentration of all ions • Observations – All SiO2 could be accounted for by dissolution of aluminosilicates, no quartz dissolution needed – Waters gain much of their SiO2 over a very short distance; action of high CO2 – Despite abundant K-feldspar, 80% of dissolved ions came from plagioclase weathering 41 Perennial springs • Can same reactions be assumed to be occurring in perennial springs? – Not necessarily – Look at ratio of ions in solution 42 Ephemeral vs. Perennial Springs SiO2 Ca Mg Na K HCO3 SO4 Cl TDS (ppm) pH ephemeral mM 0.273 0.078 0.029 0.134 0.028 0.328 0.01 0.014 perennial mM 0.410 0.260 0.071 0.259 0.040 0.895 0.025 0.03 36 6.2 75 6.8 difference mM 0.137 0.182 0.042 0.125 0.012 0.567 0.015 0.016 43 Ephemeral vs. Perennial Springs • Differences between spring types – Cl assumed to come from NaCl, SO4 from CaSO4 • Weak assumptions, but very low concentrations – SiO2:Na ratio for difference between springs is 1:1 • SiO2:Na ratio in solution for weathering of plagioclase is 2:1 – Some secondary mineral other than kaolinite being produced to remove SiO2 44 Ephemeral vs. Perennial Springs • Potential candidates for SiO2: clay mineral (smectite); amorphous SiO2 – Hypothesized reactions • Plagioclase and biotite kaolinite • Plagioclase smectite – Ended up with extra Ca and HCO3-, dissolution of CaCO3 • Potential sources of CaCO3 – Summer wet/dry deposition – CaCO3 in fracture fillings 45 Reaction Path Models • Good for simple systems where flowpaths are well defined – The larger and more complex the systems, the harder it is to constrain potential reactions • Can consider redox reactions, gas exchange, isotopic reactions, mixing of waters, etc. • N.B.: there is no unique solution – Modeler determines which phases to consider – Based on available data and “intuition” 46 Redox reactions in Groundwater • Redox reactions are extremely important in groundwater and soil water • Many key elements are redox sensitive: – C, N, S, Fe, Mn, As, heavy metals • Very important in terms of water quality/chemistry 47 Factors Controlling Natural Redox Conditions • O2 in recharge • Organic matter content of solids – Occasionally dissolved organics (natural) • Presence of redox buffers, usually minerals • Groundwater residence time 48 Groundwater Chemistry: Redox Evolution • Water tends to become more reducing as it moves along a flow path – Isolated from atmosphere, so once O2 consumed it is not replenished – Organic matter most commonly oxidized compound • Sulfide minerals can also be important – Most rapidly in the shallow zones 49 Microbes and Redox Reactions in Groundwater • Almost all redox reactions in groundwater are biogeochemically mediated – Microorganisms catalyze almost all redox reactions and use the energy released – Microbes also need a carbon source (as well as other nutrients) 50 Role of Microrganisms • Microorganisms produce enzymes that bring reactants into close proximity • Enzymes specific to substrate: carbon source and terminal electron acceptor (TEAP) (i.e., O2, NO3-, Fe(OH)3, …) – Enzyme induction: ability to create new enzymes to adapt to new carbon source (i.e., organic contaminants) • In any soil, there exists a huge variety of microorganisms but there is usually a dominant species or set of species – DNA/RNA techniques used to identify dominant species – Non-dominant species exist in isolated microenvironments • Biofilms (“slime”): “engineered” microenvironments 51 Groundwater Chemistry: Redox Evolution • Dissolved oxygen (DO) – In clayey/silty soils, DO commonly below detection in shallow groundwater – DO is generally detectable in recharge areas and in sandy soils and karstic limestones – If there is little or no soil over permeable fractured rock, detectable DO can persist far into the flow system • Occasionally an entire flow system is oxygenated 52 Organic Matter Oxidation • O2 has low solubility – 9 mg/L at 25°C (2.8 x 10-4 moles/L) – 11 mg/L at 5°C • Half reactions – OM oxidation: CH2O + H2O CO2 + 4H+ + 4e– O2 reduction: O2 + 4H+ + 4e- 2H2O – CH2O + O2 CO2 + H2O • For every mole of OM oxidized, one mole O2 of reduced • Therefore, DO typically consumed in the soil zone and shallow groundwater, resulting in anoxic groundwater 53 DO Consumption Flooded soil 54 Groundwater Chemistry: Redox Evolution • After DO is consumed, other TEAPs are used by microbes based on thermodynamics – – – – – – NO3- reduction (denitrification) MnO2 [Mn(IV)] reduction Ferric [Fe(III)] mineral reduction SO42- reduction Fermentation and methanogenesis (CO2 reduction) “Redox ladder” • The order of the reactions based on obtainable energy for the microbes • Kinetics: the less the energy, the slower the reaction 55 Role of Microrganisms • Microorganisms are subject to the laws of thermodynamics (as are we) – They catalyze reactions until equilibrium is reached (ΔG = 0) or until TEAP is consumed (reaction goes to completion) – For example, when O2 is TEAP • CH2O(aq) + O2 CO2 + H2O – When O2 is consumed and NO3- is present, denitrifying organisms have competitive advantage because they get more energy from reaction than Fe, Mn, or SO42- reducers 56 Redox Ladder: electron acceptors and donors 57 Post-DO redox reactions involving OM • Unbalanced reactions: • CH2O + NO3- CO2 + N2 (denitrification) – 5 CH2O + 4 NO3- + 4 H+ 5 CO2 + 2 N2 + 7 H2O – This reaction causes pH to increase, which is indirect evidence that denitrification has occurred • CH2O + NO3- CO2 + NH3 (ammonification; toxic to fish) 58 Post-DO redox reactions involving OM • CH2O + Fe(OH)3 CO2 + Fe2+ (iron reduction; dissolves Fe(III) minerals) – CH2O + 4Fe(OH)3 + 8 H+ CO2 + 4 Fe2+ + 11 H2O • CH2O + SO42- CO2 + H2S (sulfate reduction) – 2CH2O + SO42- + H+ 2 CO2 + HS- + 2 H2O – Water from Normal well field has a rotten egg (H2S) smell—why? High organics and sulfate-reducing bacteria active 59 Fermentation and Methanogenesis • Reactions that occur when all external electron acceptors have been used; methane (CH4) is produced, CO2 both produced and consumed • Transformation of complex organics into simpler compounds • Fermentation: CH3COOH CH4 + CO2 – CH3COOH = Acetic acid – Also produces H2 • 2 H + + 2 e- H 2 – Fermentation byproducts are used by methanogenic microbes 60 Fermentation and Methanogenesis • Methanogenesis: CO2 + 4 H2 CH4 + 2 H2O – Methane production characterized by increasing H2 – Methanogens need fermenters • H2 is a reactive intermediate product, produced and consumed by metabolic processes – Low at high Eh, higher at lower Eh – H2 is best indicator of dominant TEAP, but difficult to measure (field GC) 61 General order of microbially-mediated redox reactions Conceptual change in concentrations with time/distance 62 TEAPs in Groundwater Contaminated Uncontaminated FLOW 63 TEAPs • While thermodynamics predicts an orderly progression of the dominance of individual TEAPs, it’s not so simple in nature – Often have 2 (or more) TEAPs active in same part of aquifer • e.g., often have Fe3+ -reduction and SO42--reduction occurring together, even though Fe3+ reduction more thermodynamically favorable – Due to: micro-environments, different microorganisms responsible, solid vs. aqueous environments – Where there’s energy to be gained, microbes are working 64 TEAPs and Eh Ranges 65 Determining predominant TEAP 66 Redox Buffering • The Eh of groundwater does not linearly decline as oxidizers are consumed along a flow path • Instead, the Eh remains relatively constant as a particular oxidizer is consumed, then the Eh drops and stabilizes again 67 Redox Buffering 68 Redox Buffering • System is buffered if oxidizable or reducible compounds are present that prevent a significant change in Eh when strong oxidizing/reducing agents added – Expect Eh of natural waters to generally be in buffered ranges – Values in unbuffered ranges unstable 69 Computed vs. Measured Field Eh - Vertical bands indicate buffered ranges; reflect the standard E° 70 Redox Buffering • Example: recharging water has dissolved O2, Eh will remain high until O2 is consumed; after O2 gone, Eh drops rapidly and stabilizes at the value determined by next oxidizer • Buffers can be dissolved species or solid matter – Dissolved species: usually limited in concentration and consumed rapidly (if right conditions exist) – Solid matter: can provide large buffering capacity – E.g., Fe(OH)3 can provide buffering until equilibrium is reached with dissolved Fe concentration 71