Adrian Moore
advertisement

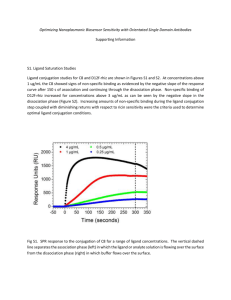
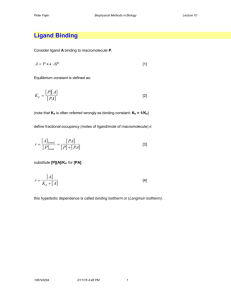
scientiam dulce hauriens Dr. Adrian Moore Department of Pharmacy, Health and Well-being Faculty of Applied Sciences UoS Sciences Complex • • • >£20 million invested in capital development programmes – new-build projects – refurbishment of existing estate – on-going developments To provide modern, well-equipped facilities to support high quality teaching and learning, real-life applied research, and support for business and our wider community – One-North East – EU ERDF (innovation, enterprise and business support) Health and Science Academy “provide the best postgraduate teaching and research facilities to accommodate the professional development needs of the pharmaceutical supply chain and support small companies and business start-up ventures in the region” Computational Methods • Comprehensive investigation of molecules, their reactions and interactions – chemical structures / biological reactions at the molecular level – discovery of new drugs that target particular cells • Pharmacophore or Quantitative Structure Activity Relationship (QSAR) – rationalise existing biological data to make informed choices as to the next series of compounds to make Rational Drug Design – good quality structural information is available of intended biological target enzyme, receptor protein or strand of DNA/RNA • • • Molecular mechanics (MM) methods Quantum mechanical (QM) methods – highly computationally resource intensive – UoS cluster computer application framework Synthesis • • Traditional; research -> medium scale – Range of current projects Flow chemistry – wide range of reactions possible – production of libraries or on multi-gram scale – safely use highly reactive/hazardous reagents – easily uses solid-phase reagents – good reproducibility -> reduced scale-up issues – inherent reaction control and selectivity – wide temperature range -> superheating gives faster reactions – reduced scale limitations -> quick reaction evaluation O O pyBOP, TEA OH X + H2 N Y O X N H X N Y N N O O OH N N X N H Y N O N H X Y OH N N N 20 compound library, synthesis and aqueous work-up, high yield and high purity (LCMS), no purification 40 mg of each compound synthesised, mass recovery 87.5 % ± 1.5 SPR – Linked to Flow Synthesis • Analyse library molecular substrate/target interactions in real time – proteins – nucleic acids – lipids and membrane-associated molecules – carbohydrates – whole cells – viruses/bacteria • Obtain a wide range of critical, binding-related data – specificity – binding partners – affinity – kinetics – concentration – thermodynamics Chip: • hydrophilic • flexible • low non-specific binding • high binding capacity • easy to activate and use for covalent attachment of ligand • withstands extensive regeneration Attachment of ligand • amine coupling • ligand thiol coupling • surface thiol coupling • maleimide coupling • aldehyde coupling Analyte Ligand buffer sample buffer association dissociation Separation Science: Areas of Expertise • All areas of pharmaceutical and biomedical analysis – medicinal plant extracts – biomarker analysis – cleaning validation – drug bioanalysis – preparative isolation of API – preparative isolation of related substances – rapid API screens / related substances – chiral screening – stability screening – confirmation of structure – unknown identification Analytical Science: Capability • • • • • HPLC / UHPLC (better efficiency, high throughput) • ELSD (non volatile, nonchromaphoric compounds) • RI (more volatile, nonchromaphoric compounds) • fluorescence • diode array • single quad MS cooled auto samplers on MS (to 4C) • UHD-QTOF (high resolution and mass accuracy) • prep-LC GC-FID, GC-Q, GC-QQQ • SPME, head space, and cold-on-column MALDI-TOF CE, CE-MS • proteins and complex/biological analysis especially atomic absorption, flame photometry, UV, IR, fluorometry, luminometry, Karl Fischer, ionchromatography, logD, pKa, TGA-DSC, microscopy (SEM, TEM, confocal) Separation Science: Rapid LC Related Substances Method UPLC of paroxetine and all related substances in 1.2 minutes BEH C18 (1.7 mm) (50 mm x 2.1 mm ID); 1 mg ml-1 paroxetine and related substances at ~ 0.002 mg ml-1. UV detection - 295 nm. Mobile phase component A (water – THF – TFA (90:10:0.5, v/v/v)), B (acetonitrile – THF – TFA (90:10:0.5, v/v/v)). Resolution was maintained when using a steep gradient profile throughout and also when the temperature was raised to 80 oC. Complex Samples Unknown identification tmsi glu , 11-Dec-2009 + tmsiglu 561 (5.457) Rf (7,5.000) 73.0274 573980672 100 140.1913 415662080 98.0790 338937856 125.0983 301457408 % 70.0108 58.9439 61574144 31477320 74.0328 50367488 95.0674 7497911 99.0827 38347776 59 69 79 89 99 109 119 141.1304 58639360 126.1006 35997696 73 100 0 49 112.1263 7868672 129 213.1751 2671328 139 149 159 169 179 189 199 209 219 265.0 6003 229 249 259 73 100 98 140 98 125 50 140 Si 50 N 125 0 239 55 62 50 60 70 70 84 80 109 90 100 110 213 120 C:\ TurboMass\ Default.pro\ Data\ tmsiglu: Scan 561 (0. min) 130 140 150 160 170 180 190 200 210 Side by Side MF=944 RMF=968 265 220 230 240 250 260 N 270 1H-Imidazole, 1-(trimethylsilyl)- 70 55 0 50 84 63 60 70 80 112 90 100 110 120 130 140 150 160 170 180 190 200 210 220 230 240 250 Complex Samples – Principle Analysis • Metabolic profiling of low MW components in biological fluid samples • Pathological conditions can create metabolic disruptions detectable in the metabolite content of biofluids • variation in concentration and relative proportion • Identify affected pathway -> novel approach to treatment NMR Services • • • Structure confirmation / determination – spectra with or without interpretation – cooled auto-sampler for biological/unstable samples – fast turnaround routine service Impurity profiling / identification by NMR / MS Characterisation of peaks in liquid chromatograms, LC-NMR – characterisation of unstable components in complex mixtures – structure elucidation of minor components in complex mixtures – detailed structural information where LC-MS is inappropriate Identification of Natural Products : Comparison Extracted Product/Standard Extract High resolution MS : M = 610 g mol-1, C27H30O16 1-D NMR 500 MHz LC-1H NMR spectrum of approx. 2 mg of rutin, isolated from extracts of Sophora japonica , in D2O-CH3CN [solvent suppression at d1.90 (CH3CN) and d4.46 (residual water)] Standard S 24795 S 24795 completed Phase I Clinical Studies Synthesis developed to semi-production scale (500 kg) negative allosteric modulator at nicotinic receptors wide-spectrum pro-cognitive, psycho-behavioural activity Electrophysiology a7 : 42 ± 2 mM a4b2 : 230 ± 16 mM (rat – expressed on Xenopus Laevis oocytes) Binding studies a7 > 10000 nM, a4b2 > 10000 nM (a1)2bdg ~ 10000 nM, a3b4 > 10000 nM security binding (80 sites) – no negative interactions CaCo2 100% hCMEC/D3 90% Microsomes rat 31%, human 75% in vitro clastogenotoxicity! (minor metabolite related, < 0.2% profile) Identification of Metabolite: Extraction From Rat Bile Parent compound Aromatic CH NH Metabolite ; 352 scans (20min) 2 µg MS analysis : +16 → oxidation MS/MS → oxidation on one of the 3 substituted aromatic rings exact position of hydroxylation from LC-NMR WEEK 1 Pharmaceutics • Pre-formulation studies – phase transitions / microscopic changes – polymorphic form studies – solubility screening – pKa, log P/D determination – solution and solid-state stability, degradation studies, degradation product identity – excipient and active compatibility studies – particle size measurements – powder electrostatics – physical characterisation of drug delivery systems and vehicle optimisation – problem solving of existing processes and formulations • Formulation – tablet, capsule, solution, suspension, emulsion, injectable, patch etc. – protein stabilisation and delivery • Aseptic capability – category 2 clean room Dr. Adrian Moore Department of Pharmacy, Health and Well-being Faculty of Applied Sciences University of Sunderland Dale Building, Room 1.03 Sciences Complex Wharncliffe Street Sunderland SR1 3SD T : +44 (0)191 515 2554 F : +44 (0)191 515 3405 E : adrian.moore@sunderland.ac.uk