Molecular Covalent
advertisement

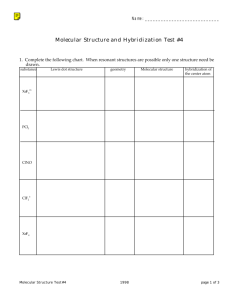
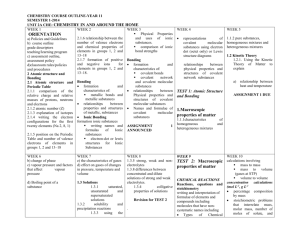
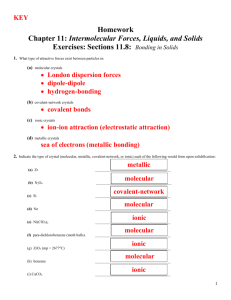
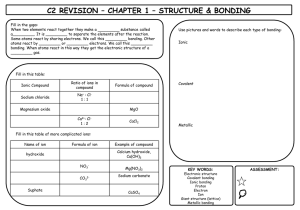
Molecular covalent Network covalent Ionic Metallic Let’s look at the table! Type Structure Hardness Melting/ boiling point Conductivity Molecular covalent Click Click Click Click Network covalent Click Click Click Click Ionic Click Click Click Click Metallic Click Click Click Click Back to table Crystal lattice structure: alternating cations and anions held together by electrostatic attractions (+ attracted to -, just like a magnet!) Ionic compounds are hard!! Make sure in your table, you also know what it means that an ionic compound is brittle and why! http://auden.webster.edu/~scottsus/characteri stics.htm Back to table Back to table http://www.kentchemistry.com/links/bonding/ ionicProps.htm Back to table Read the caption for the three pictures in the link below. http://www.chem.ufl.edu/~itl/2051_s97/week _1/ionic.html Back to table http://sixthsense.osfc.ac.uk/chemistry/bondin g/metallic.asp Back to table Scroll down to “Strength and workability” and write information about hardness, malleability, and ductility http://www.chemguide.co.uk/atoms/structure s/metals.html#top Back to table Scroll to slide 8!! http://www.slideshare.net/chemistryhelpline/p roperties-of-compounds-ionic-covalentand-metallic Back to table Scroll to slide 8!! http://www.slideshare.net/chemistryhelpline/p roperties-of-compounds-ionic-covalentand-metallic http://www.avogadro.co.uk/structure/chemstr uc/network/g-molecular.htm Click on the link, and press “covalently bond C atoms” and “keep bonding” to see the structure. Back to table http://www.avogadro.co.uk/structure/chemstr uc/network/g-molecular.htm Read the first paragraph! Back to table Scroll to slide 4!! http://www.mi.mun.ca/users/pfisher/Chemistr y1011_92.pdf Back to table Since there is a network of strong covalent bonding, network covalent solids are very hard. Back to table Molecular covalent are all nonmetal atoms. You drew examples of these when we did lewis structures. The molecules are held together by intermolecular forces. Here is an example: Back to table Since molecular covalent molecules are held together by IMFs which are MUCH weaker than bonds, molecular covalents are considered soft molecules. Back to table Scroll a ways down to “electrical conductivity” http://www.chemguide.co.uk/atoms/structure s/molecular.html Back to table 2nd paragraph!! http://www.chemguide.co.uk/atoms/structure s/molecular.html Back to table