Constraining the effects of Mg:Ca ratio and temperature on
advertisement
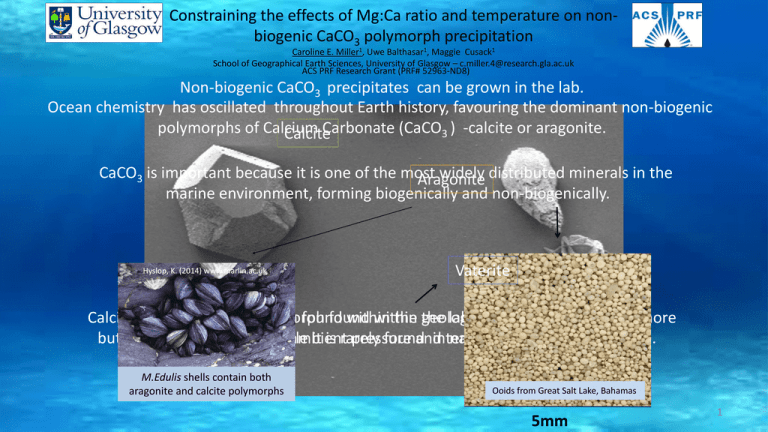
Constraining the effects of Mg:Ca ratio and temperature on nonbiogenic CaCO3 polymorph precipitation Caroline E. Miller1, Uwe Balthasar1, Maggie Cusack1 School of Geographical Earth Sciences, University of Glasgow – c.miller.4@research.gla.ac.uk ACS PRF Research Grant (PRF# 52963-ND8) Non-biogenic CaCO3 precipitates can be grown in the lab. Ocean chemistry has oscillated throughout Earth history, favouring the dominant non-biogenic polymorphs of Calcium Carbonate (CaCO ) -calcite or aragonite. 3 Calcite CaCO3 is important because it is one of the most widely distributed minerals in the Aragonite marine environment, forming biogenically and non-biogenically. Hyslop, K. (2014) www.marlin.ac.uk Vaterite One otherare polymorph found within laboratory setting, Calcite and aragonite both found within the the geological record, but calcite is more but because it is very unstable it is rarely found intemperature. natural conditions, is vaterite. stable at ambient pressure and M.Edulis shells contain both aragonite and calcite polymorphs Ooids from Great Salt Lake, Bahamas 5mm 1 CaCO3 precipitation experiments were designed Fluctuations into theinvestigate seawater Mg:Ca ratio may cause shifts in original composition of non-biogenic marine and temperature Still carbonates toonbenon-biogenic dominated by either calcite or aragonite (Morse et al., 2007) . Higher temperature increases the growth rate of aragonite , while calcite growth slows solution CaCO3 (based on Morse et al., 2007 & (Burton & Walter, 1987). Bots et al., 2011). Temperature within seawater changes latitudinally. Constant addition of NaHCO3 to Shaken solutions of known Mg:Ca Therefore, the spatial distributiono of polymorph formation may be influenced by bothsolution ratio (1, 2 &3) were carried out at 20 C temperature and Mg:Ca ratio. o & 30 C in still and shaken conditions (shaking to mimic the natural environment). Sandberg (1983) proposed an ‘aragonite threshold’ where Question: below Mg:Ca ratio of 2 only calcite will precipitate ; above 2, aragonite also precipitates. What is the effect of combining temperature and Mg:Ca ratio on CaCO3 polymorphs? CaCO3 precipitates were analysed using ‘Aragonite-calcite seas’ are viewed as a global phenomenon where Raman Spectroscopy and conditions fluctuate over time. This does not consider latitudinal temperature variations . Scanning Electron Microscope (SEM). 2 Influence of water movement (shaking conditions) Results are presented for the number of crystals precipitated as proportions of CaCO However, temperature further influences these proportions of crystals grown. 3 These results show that increased Mg:Ca ratio influences the polymorph proportions precipitated. o Influence of temperature (still conditions) Increased Mg:Ca ratio on CaCO precipitates (still conditions) resulting from increased Mg:Ca ratios of 1,3 2 & 3, and temperature s of 20 & 30 C. Fewer calcite & aragonite crystals are precipitated More crystals precipitate in still conditions than Therefore, Mg:Ca ratio cannot be investigated in isolation without considering the influence of The Insame trends in CaCO polymorphs caused by Mg:Ca ratio are also present when temperature is 3 all experiments calcite, aragonite and vaterite polymorphs were found to co-precipitate Numbers of calcite crystals decrease in shaken conditions. shaken. temperature. increased. at all Mg:Ca ratios (1, 2 & 3). o but more aragonite and vaterite crystals precipitate at 30 C. More vaterite crystals precipitate in shaken conditions. In order to mimic the natural environment, all experimental scenarios were repeated Numbers of vaterite crystals are minor compared to The number of aragonite and These findings are based on non-biogenic precipitates. Numbers of calcite crystals with the addition of water movement. Fewer crystals precipitate Fewer crystals precipitate in total at numbers of calcite and aragonite. vateriteatcrystals precipitated precipitated decrease at higher Mg:Ca higher Mg:Ca higher temperatures at higher Mg:Ca. ratios However, as these results demonstrate non-biogenicincrease polymorphs can be influenced by temperature, these findings can be applied to biogenic polymorph forms. Considering temperature alongside Mg:Ca ratio in a range of conditions that mimic the natural environment allows a realistic framework which can be applied to conditions today. Biomineralising organisms live within these seawater conditions therefore could influence the subsequent biomineralisation that occurs. References • • • • • Bots , P., Benning, L.G., Rickaby, R.E.M. & Shaw, S. (2011) The role of So4 in the switch from calcite to aragonite seas. Geology. 39, 331-334. Burton, E.A. & Walter, L. M. (1987) Relative precipitation rates of aragonite and Mg-calcite from seawater: Temperature or carbonate ion control? Geology. 15, 111-114. Morse, J.W., Arvidson, R.S. & Luttge, A. (2007) calcium carbonate formation and dissolution. Chemical Reviews. 107, 342-381. Sandberg, P.A. (1983) An oscillating trend in Phanerozoic non-skeletal carbonate mineralogy. Nature. 305 (1), 19-22. Stanley, S.M. & Hardie, L.A. (1998) Secular oscillations in the carbonate mineralogy of reef-building and sediment-producing organisms driven by tectonically forced shifts in sediment3 producing organisms driven by tectonically forced shifts in seawater chemistry. Palaeogeography, Palaeoclimatology, Palaeoecology. 144, 3-19.