Strong and Weak Acids and Bases
advertisement

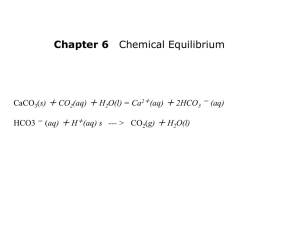
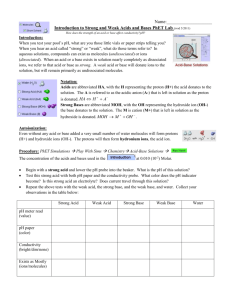
Strong and Weak Acids and Bases Pg 560-578 The strength of an acid is determined by the extent to which it ionizes, its percent ionization, not the concentration of the acid, the concentration of its hydronium ions, or its ability to react with a metal Strong Acid An acid that nearly completely dissociates All molecules of the acid break up to form the ions soluble in water If more than one proton is being removed, not all steps need to be complete dissociation. Weak Acid An acid that only slightly dissociates in a water solution Only a small percent of acid molecules donate their hydrogen, and most remain the same. Example: CH3COOH A strong acid essentially ionizes 100%. An example of a strong acid is hydrochloric acid, HCl (aq) HCl(g) + 0.10 mol H2O(l) H3O+(aq) + Cl-(aq) 0.10 mol 0.10 mol 100% ionization few molecules many ions An example of a weak acid is acetic acid, CH3COOH. CH3COOH(l) + H2O(l) H3O+(aq) + CH3COO-(aq) 0.10 mol << 0.10 mol << 0.10 mol 5% ionization at 25C many molecules few ions Strong Base A base that dissociates almost completely into its ions. All oxides and hydroxides of group 1 and 2 are strong bases. Ex: NaOH Weak Base Most bases are weak They dissociate only slightly in a water solution Example: NH3 Strong acids are strong electrolytes and weak acids are weak electrolytes A strong base dissociates 100%. An example of a strong base is sodium hydroxide, NaOH. NaOH(s) + H2O(l) Na +(aq) + OH-(aq) 0.10 mol 0.10 mol 0.10 mol 100% dissociation few formula units (NaOH) many ions A weak base ionizes to a small extent. An example of a weak base is NH3(g). NH3(g) + H2O(l) NH4+(aq) + OH-(aq) 0.10 mol << 0.10 mol << 0.10 mol 5% ionization at 25C many molecules few ions Strong bases are strong electrolytes and weak bases are weak electrolytes. Examples of Strong Acids and Bases Strong Acids Strong Bases HClO4 perchloric acid HCl hydrochloric acid HNO3 nitric acid H2SO4 sulfuric acid HBr hydrobromic acid HI hydriodic acid LiOH lithium hydroxide NaOH sodium hydroxide KOH potassium hydroxide RbOH rubidium hydroxide CsOH cesium hydroxide Ca(OH)2 calcium hydroxide Sr(OH)2 strontium hydroxide Ba(OH)2 barium hydroxide To experimentally distinguish strong acids from weak acids; and strong bases from weak bases: compare a strong acid to a weak acid of equal concentration – more hydronium ions and anions will be present in the strong acid solution compare a strong base with a weak base of equal concentration – more hydroxide ions and cations will be present in the strong base solution Therefore, we could compare a strong acid and a weak acid of equal concentration by: a) use a conductivity apparatus test (light bulb will be brighter for a strong acid). b) measure conductivity of solutions (strong acid will have a higher conductivity). c) react the two acids with a metal like magnesium (stronger acid will react faster, more bubbling as H2 is formed) d) measure the pH of the solutions using a pH meter or indicators (strong acid has a lower pH) A strong base can be distinguished experimentally from a weak base of equal concentration by: a) use a conductivity apparatus test (light bulb will be brighter for a strong base) b) measure conductivity of solutions (strong base will have a higher conductivity) c) react the two bases with a chemical and observe the rate of the reaction (stronger base will react faster) d) measure the pH of the solutions using a pH meter or indicators (stronger base has a higher pH) Dissociation Equation A balanced chemical equation showing all ions produced when an ionic compound dissolves Example: HSO4-(aq) + H2O(l) SO42-(aq) + H3O+(aq) Acids: Concentration vs. Strength WEAK STRONG CONCENTRATED H+ A- H+ A- H+ A- H+ A- HA A- H+ A- H+ A- H+ A- H+ A H+ A- HA H+ A- H+ A- H+ AA- H+ A- H+ A- H+ A- H+ A- H+ H+ A - H + A - H + A - HA H + A A- H+ A- H+ A- H+ A- H+ A– H+ A- H+ A- H+ A- H+ A- H+ A- H+ A- H+ A- H+ A- H+ AHA A- H+ A- H+ A- H+ A- H+ HA HA H+ A- HA HA HA HA HA HA HA H+ A- HA HA HA HA HA HA H+ A- HA HA HA HA HA H+ A- HA H+ A- HA HA HA HA HA HA HA H+ A- HA H+ A- HA HA HA HA HA HA H+ A- HA HA HA H+ AHA HA HA HA HA HA HA DILUTE H+ A- H+ A- HA A- H+ A- H+ A– H+ A- H+ A- H+ A- H+ HA H+ A- HA H+ HA A- HA HA HA H+ A - HA HA HA HA H+A– A- Dissociate nearly 100% HA H1+ + A- + A- H+ HA HA HA H+A– HA STRONG ACIDS HA WEAK ACIDS Dissociate very little HA H1+ Strong vs. Weak Acid Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 508 Comparing Strengths Tables tend to list strong acids towards the top, and strong bases towards the bottom Figure 14.12 in text page 563 Strengths Of Conjugate Acid-Base Pairs The stronger an acid, the weaker is its conjugate base. The stronger a base, the weaker is its conjugate acid. An acid-base reaction is favored in the direction from the stronger member to the weaker member of each conjugate acidbase pair. Concentrated vs. Dilute 0.3 M HCl 10.0 M CH3COOH Dilute, strong acid Concentrated, weak acid 2.0 M HCl Concentrated, strong acid OR Dilute, strong, acid 12.0 M HCl Concentrated, strong acid Review and Practice Page 558-559 # 1-2,4, 6-11