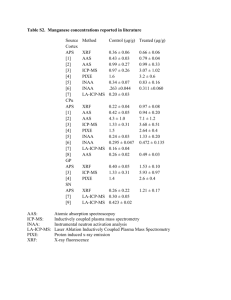
Dissolved Oxygen Kit Reactions

http://www.lamotte.com/images/pdf s/instructions/7414.pdf
Step 1:
Manganous (Manganese II) Sulfate (Manganous Sulfate
Solution) reacts with Potassium Hydroxide (Alkaline Potassium
Iodide Azide Solution [KOH & KI]) to form Manganous
(Manganese II) Hydroxide and Potassium Sulfate.
MnSO
4
+ 2KOH ⎯⎯→ Mn(OH)
2
+ K
A precipitate is formed…
2
SO
4
Mn(OH)
2 (s)
Step 2:
Manganous (Manganese II) Hydroxide reacts with Oxygen &
Water to produce Manganic (Manganese III) Hydroxide, (a redox reaction). The ratio of oxygen to Manganese II
Hydroxide is 1:4.
4Mn(OH)
2 (s)
+ O
2 (aq)
+ 2H
2
O
(l)
⎯⎯→ 4Mn(OH)
3 (s)
Product is brown precipitate.
Step 3:
Manganic (Manganese III) Hydroxide reacts with Sulfuric Acid to produce Manganic (Manganese III) Sulfate and Water.
2Mn(OH)
3 (aq)
+ 3H
2
SO
4 (aq)
⎯⎯→ Mn
2
(SO
4
)
3 (s)
+ 6H
Oxygen is considered “fixed” at this point.
2
O
(l)
The previous reaction is immediately followed by Manganic
(Manganese III) Sulfate reacting with Potassium Iodide (from the step 1 addition of Alkaline Potassium Iodide Azide
Solution) to form Manganous (Manganese II) Sulfate and
Potassium Sulfate and Iodine. (another redox reaction)
Mn
2
(SO
4
)
3 (s)
+ 2KI
(aq)
⎯⎯→ 2MnSO
4 (aq)
+ K
2
SO
4 (aq)
+ I
2 (aq)
The iodine causes the solution to appear yellow-brown. The amount of iodine is proportional to the amount of oxygen in
Step 2.
Step 4:
Sodium Thiosulfate (S
2
Sodium Tetrathionate (S
O
4
3
-2
O
) reacts with the Iodine to form
6
-2 ) and Sodium Iodide.
2Na
2
S
2
O
3 (aq)
+ I
2 (aq)
⎯⎯→ Na
2
S
4
O
6 (aq)
+ 2NaI
(aq)
When all of the iodine has finished reacting, the solution changes color from yellow-brown to colorless.
Starch indicator may be added to enhance this endpoint. It will form a blue-black complex with iodine (not iodide ion) when present.