Representing Chemical Reactions
advertisement

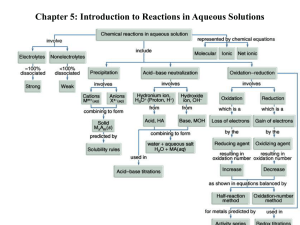
Temperature change Release of energy (heat or light) Color Change Odor Gas Bubbles All chemical reactions have two parts: Reactants and Products. Reactants Products Example: aluminum(s) + bromine(l) aluminum bromide(s) Example: aluminum(s) + bromine(l) aluminum bromide(s) Skeleton equation: Al (s) + Br2 (l) AlBr3 (s) Hydrogen and bromine gases react to yield hydrogen bromide. hydrogen (g) + bromine (g) hydrogen bromide (g) H2 (g) + Br2 (g) HBr (g) Carbon monoxide and oxygen react to form carbon dioxide. carbon monoxide (g) + oxygen (g) carbon dioxide (g) CO (g) + O2 (g) CO2 (g) Step 1: Write a skeleton equation from a word equation Step 2: Count all of the atoms in the reactants Hydrogen (g) + Chlorine (g) H2 (g) + Cl2 (g) HCl (g) H2 (g) + Cl2 (g) HCl (g) 2 2 Step 3: Count number of atoms in products H2 (g) + Cl2 (g) HCl (g) 2 atoms total Step 4: Change the coefficients to make the number of atoms of each element equal on both sides of the equation. H2 (g) + Cl2 (g) 2HCl 2 2 4 Write a balanced chemical equation for the reaction in which aqueous sodium hydroxide and aqueous calcium bromide produce solid calcium hydroxide and aqueous sodium bromide. NaOH(aq) + CaBr2(aq) Ca(OH)2(s) + NaBr(aq) How many atoms in the reactants? How many atoms in the products? 5 7 Balanced equation: 2NaOH(aq) + CaBr2(aq) Ca(OH)2 + 2NaBr(aq) Synthesis Combustion Decomposition Replacement (Single-Replacement, DoubleReplacement) Two reactants form one product It can either be two elements or two compounds reacting to form a new compound. Example: A + B AB CaO(s) + H2O(l) Ca(OH)2 An element and a compound can also combine Example: SO2(g) + O2(g) SO3(g) 2SO2(g) + O2(g) 2SO3(g) This is when a compound breaks down into two or more elements or other compounds AB A + B Example: NaN3(s) Na(s) + N2(g) Balanced? 2NaN3(s) 2Na(s) + 6N2(g) A compound reacts with an element and one element replaces one of the atoms. A + BX AX + B Example: Li(s) + H2O(l) LiOH(aq) + H2(g) 2Li(s) + 2H2O(l) 2LiOH + H2(g) AX + BY AY + BX Example: Ca(OH)2(aq) + HCl(aq) CaCl2(aq) + H2O (l) Balanced? Ca(OH)2(aq) + 2HCl(aq) CaCl2(aq) + 2H2O (l) In combustion reactions Oxygen combines with a substance and releases energy in the form of heat and light. (FIRE) A common example of this is burning coal C(s) + O2 (g) CO2 Another common example is the combustion of hydrogen to make water. H2(g) + O2(g) H2O (g) 2H2(g) + O2(g) 2H2O(g) An aqueous solution contains one or more dissolved substances called solutes. In an aqueous solution the solvent is water. In an aqueous solution of Hydrocholric acid… HCl(aq) H+(aq) + Cl-(aq) In an aqueous solution of sodium hydroxide NaOH(aq) Na+(aq) + OH-(aq) 1. All salts of Group IA, and ammonium are soluble. 2. All salts of nitrates, chlorates and acetates are soluble. 3. All salts of halides are soluble except those of silver(I), copper(I), lead(II), and mercury(I). 4. All salts of sulfate are soluble except for barium sulfate, lead(II) sulfate, and strontium sulfate. 5. All salts of carbonate, phosphate and sulfite are insoluble, except for those of group IA and ammonium. 6. All oxides and hydroxides are insoluble except for those of group IA, calcium, strontium and barium. 7. All salts of sulfides and insoluble except for those of Group IA and IIA elements and of ammonium. Some reactions that occur in aqueous solution produce solid precipitates. For example… 2NaOH(aq) + CuCl2(aq) 2NaCl(aq) + Cu(OH)2(s) An ionic equation shows in detail the ions that exist in the solution… Write the ionic equations and the net ionic equation for the reactions… Ba(NO3)2(aq) + Na2CO3(aq) BaCO3(s) + NaNO3(aq) Write the chemical, complete ionic, and net ionic equations for the reaction between aqueous solutions of barium nitrate and sodium carbonate that forms the precipitate barium carbonate. Acid-Base neutralizations are usually double replacement reactions that result in the formation of water. In general an acid is a compound that, when dissolved in water produces H+ ions. Examples – HCl, HBr, H2SO4 In general a base is a compound that, when dissolved in water produces OH- ions. Examples: NaOH Complete ionic equation: H+(aq) + Br-(aq) + Na+(aq) + OH-(aq) Na+(aq) + Br-(aq) + H2O(l) Net ionic equation: H+(aq) + OH-(aq) H2O(l) Write a chemical, complete ionic, and net ionic equations for the reaction between hydrochloric acid and aqueous lithium hydroxide. This reaction produces water and aqueous lithium chloride. Aqueous barium chloride and aqueous sodium fluoride are mixed. Aqueous copper (I) nitrate and aqueous potassium sulfide are mixed Aqueous sodium hydroxide and aqueous copper (II) chloride are mixed. Aqueous sodium carbonate and aqueous calcium nitrate are mixed. Aqueous sodium sulfate and aqueous potassium carbonate are mixed.