Experiment: Acid-Base Titration of Aspirin
advertisement
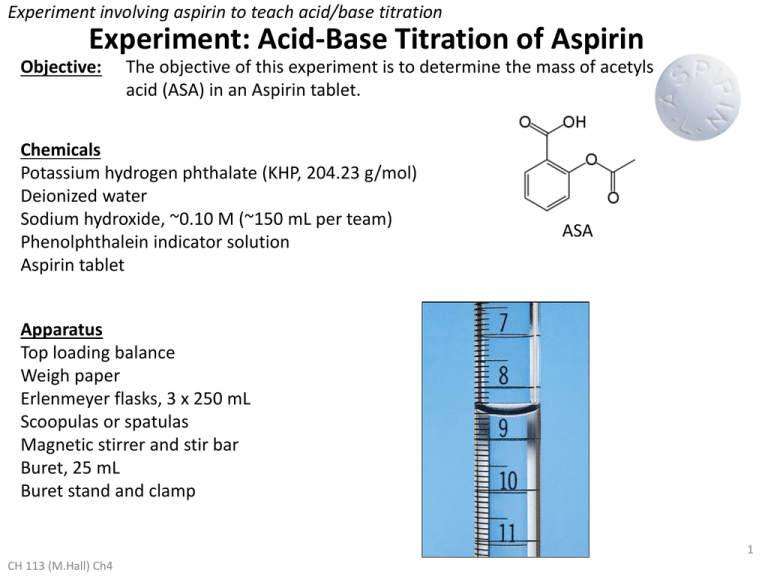
Experiment involving aspirin to teach acid/base titration Experiment: Acid-Base Titration of Aspirin Objective: The objective of this experiment is to determine the mass of acetylsalicylic acid (ASA) in an Aspirin tablet. Chemicals Potassium hydrogen phthalate (KHP, 204.23 g/mol) Deionized water Sodium hydroxide, ~0.10 M (~150 mL per team) Phenolphthalein indicator solution Aspirin tablet ASA Apparatus Top loading balance Weigh paper Erlenmeyer flasks, 3 x 250 mL Scoopulas or spatulas Magnetic stirrer and stir bar Buret, 25 mL Buret stand and clamp 1 CH 113 (M.Hall) Ch4 Data & Observations: NaOH (wet & sticky) Part I. Standardization of the Sodium Hydroxide Solution KHP (nice & dry) Solid sodium hydroxide cannot be massed accurately because it absorbs water and carbon dioxide from the air. Consequently, it is not possible to make an aqueous solution to a very specific and accurate concentration. Before you use NaOH(aq) to titrate Aspirin, you need to “standardize“ it, that is determine its accurate concentration. The solution of NaOH(aq) that you are given is approximately 0.10 M. You will titrate it against a known amount of an acid to determine the concentration of NaOH. The acid used to standardize the concentration of NaOH is potassium hydrogen phthalate (KHP). It can be massed very accurately. KHP is actually the potassium salt of the phthalate ion: KHP phthalate ion (acid) The phthalate ion is the acid that is titrated by NaOH in the standardization: CH 113 (M.Hall) Ch 4&15 2 Experiment: Acid-Base Titration of Aspirin 1. Obtain an exact mass of KHP (should be about 0.3 g) in a 250 mL Erlenmeyer flask. 2. Add approximately 100 mL of distilled water, the stir bar, and 2 drops of phenolphthalein. 3. Place the flask on the magnetic stirrer and start stirring to dissolve the acid and mix in the indicator. 4. Place a clean buret in a stand and make sure it is straight. Pour the ~0.10 M NaOH solution carefully into the buret, filling to past the top marking. 5. Place a waste beaker under the buret and dispense some NaOH to remove any bubbles from around the stopcock. Make sure the meniscus is on or below the 0 mark, but do not spend time trying to get it to exactly 0.00. 6. Record the initial volume. Check with your instructor that you have the correct sig figs. 7. Do a calculation to estimate the approximate volume of base that will be required to reach the endpoint in this first titration. Check this value with your instructor before you proceed. 8. Proceeding slowly for the first trial, titrate the KHP with the sodium hydroxide until the endpoint is reached. You should observe the appearance of a permanent pink color based on the addition of a single drop. Record the final volume of the base. 9. Calculate the concentration of the sodium hydroxide. 10. Repeat the above procedure twice, except omit the estimation of volume (why?). You may titrate more quickly since you can approximate where the endpoint will be. Be sure to slow down when approaching the endpoint. 11. Obtain three NaOH concentrations that are close enough such that the relative standard deviation (RSD) is less than 3%. s RSD = CH 113 (M.Hall) Ch 4&15 x ´100% 3 Part II. Titration of Aspirin with Sodium Hydroxide 12. Place an Aspirin tablet in a clean 250 mL Erlenmeyer flask. Add approximately 100 mL of distilled water, the stir bar, and 2 drops of phenolphthalein. The Aspirin tablet will not dissolve completely, but you can titrate it anyway. 13. Fill the buret with the NaOH solution (standardized at this point) and titrate as before, proceeding slowly. Calculations: 1. Show your calculations for the concentration of sodium hydroxide, the mean, the standard deviation, and the RSD. 2. Calculate the mass of ASA in the Aspirin tablet. 3. Assuming 325 mg ASA/tablet is the accepted value, determine your percent error. Results: Summarize your results here. Error and its Source: Analyze your error according to our usual method. CH 113 (M.Hall) Ch 4&15 4