Lab 6
advertisement
Determination of a Ksp Using Spectrophotometry Lab 6 Outline Purpose Solubility Experiment Reaction and equilibrium expression Safety Concerns Waste Spring Break and Next Lab Reminder Purpose The Ksp for the insoluble compound, copper (II) tartrate will be determined using a spectrophotometric method. The experiment will provide the student with more experience in completing serial dilutions and equilibrium calculations. Solubility The solubilities of solutes are different in different solvents because of the “like dissolves like” principle. Other factors that affect solubility are temperature and the common ion effect. Even insoluble compounds dissolve to a small extent. Ksp is a measure of the solubility of solutes. Solubility The larger the Ksp, the greater the solubility; the smaller the Ksp, the smaller the solubility. Ksp of soluble and insoluble compounds can be determined spectrophotometrically. Factors that affect the amount of light absorbed by a compound are concentration of the absorbing species, path length through the solution and molar absorptivity of the light absorbing species, per Beer’s Law. Experiment The copper species in CuSO4 is the same as the copper species in CuC4H4O6. The saturated CuC4H4O6 solution we will be analyzing has an absorbance value that is already very low. Dilutions are out of the question. We can therefore construct a calibration curve using dilutions of CuSO4, since Cu2+ absorbs light of a 775 nm wavelength and SO42- is colorless. Experiment Measure the absorbance of Cu2+(aq) at different concentrations to make up a calibration curve of Abs vs. [Cu2+], M. This calibration curve can be used to determine the concentration, and therefore solubility, of any Cu2+(aq) solution, if the unknown solution has an absorbance that falls in the same absorbance range as our calibration curve. The absorbance can be measured for copper (II) tartrate to determine its solubility, based on the equation of the calibration curve of Cu2+(aq) (with appropriate significant figures) and the stoichiometric dissociation ratio of copper tartrate. Reaction and Equilibrium Expression CuC4H4O6(s) Cu2+(aq) + C4H4O62-(aq) Ksp = [Cu2+] [C4H4O62-] (Remember that solids are not included in the equilibrium expression!) Since copper (II) tartrate dissociates in a 1:1 ratio, it’s easy to determine the Ksp once [Cu2+] is found using a calibration curve. Safety Concerns Reagents: Cupric sulfate Copper (II) tartrate Eye Contact: Irritation, conjunctivitis, ulceration, clouding of cornea Skin Contact: Irritation and itching Inhalation: Coughing, sore throat, shortness of breath, ulceration and perforation of the respiratory tract. Fumes from heating may cause symptoms similar to a cold. Ingestion: Burning of the mouth, esophagus, and stomach. Hemorrhagic gastritis, nausea, vomiting, abdominal pain, metallic taste, and diarrhea. Systemic copper poisoning with capillary damage, headache, cold sweat, weak pulse, kidney and liver damage, CNS excitation and depression, jaundice, convulsions, blood effects, paralysis, coma and death. Waste Copper is toxic and must be disposed of in the appropriate container in the fume hood. Be sure to return your prealiquoted copper tartrate solutions to the same place you found them. DO NOT DISCARD THESE! Next Week: Spring Break No labs next week In 2 weeks: Lab 7 Reminder Lab 7 in 2 weeks.






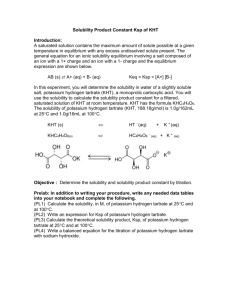














![K sp = [Pb 2+ (aq)][Cl](http://s2.studylib.net/store/data/005788724_1-fd79e2539544b4374a3f7aa03b8a844b-300x300.png)