Carey Chapter 8 Nucleophilic Sub
advertisement

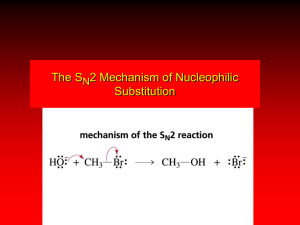
Chapter 8 Nucleophilic Substitution Dr. Wolf's CHM 201 & 202 8-1 Functional Group Transformation By Nucleophilic Substitution Dr. Wolf's CHM 201 & 202 8-2 Nucleophilic Substitution – Y: + R X Y – R +: X nucleophile is a Lewis base (electron-pair donor) often negatively charged and used as Na+ or K+ salt substrate is usually an alkyl halide Dr. Wolf's CHM 201 & 202 8-3 Nucleophilic Substitution Substrate cannot be an a vinylic halide or an aryl halide, except under certain conditions to be discussed in Chapter 12. X C Dr. Wolf's CHM 201 & 202 C X 8-4 Table 8.1 Examples of Nucleophilic Substitution Alkoxide ion as the nucleophile R' ..– O: .. + .. O .. R R X gives an ether R' Dr. Wolf's CHM 201 & 202 + :X – 8-5 Example (CH3)2CHCH2ONa + CH3CH2Br Isobutyl alcohol (CH3)2CHCH2OCH2CH3 + NaBr Ethyl isobutyl ether (66%) Dr. Wolf's CHM 201 & 202 8-6 Table 8.1 Examples of Nucleophilic Substitution Carboxylate ion as the nucleophile O ..– + R X R'C O: .. gives an ester O R'C Dr. Wolf's CHM 201 & 202 .. O .. R + :X – 8-7 Example O CH3(CH2)16C OK + CH3CH2I acetone, water O CH3(CH2)16C O CH2CH3 + KI Ethyl octadecanoate (95%) Dr. Wolf's CHM 201 & 202 8-8 Table 8.1 Examples of Nucleophilic Substitution Hydrogen sulfide ion as the nucleophile H ..– S: .. + H .. S .. R R X gives a thiol Dr. Wolf's CHM 201 & 202 + :X – 8-9 Example KSH + CH3CH(CH2)6CH3 Br ethanol, water CH3CH(CH2)6CH3 + KBr SH 2-Nonanethiol (74%) Dr. Wolf's CHM 201 & 202 8-10 Table 8.1 Examples of Nucleophilic Substitution Cyanide ion as the nucleophile :N – C: + C R R X gives a nitrile :N Dr. Wolf's CHM 201 & 202 + :X – 8-11 Example NaCN + Br DMSO CN + NaBr Cyclopentyl cyanide (70%) Dr. Wolf's CHM 201 & 202 8-12 Table 8.1 Examples of Nucleophilic Substitution Azide ion as the nucleophile – :N .. – : N .. + gives an alkyl azide + – :N N N .. .. R Dr. Wolf's CHM 201 & 202 + N R + X :X – 8-13 Example NaN3 + CH3CH2CH2CH2CH2I 2-Propanol-water CH3CH2CH2CH2CH2N3 + NaI Pentyl azide (52%) Dr. Wolf's CHM 201 & 202 8-14 Table 8.1 Examples of Nucleophilic Substitution Iodide ion as the nucleophile ..– : ..I: + R X gives an alkyl iodide .. : ..I Dr. Wolf's CHM 201 & 202 R + :X – 8-15 Example CH3CHCH3 + NaI Br acetone CH3CHCH3 + NaBr I Dr. Wolf's CHM 201 & 202 NaI is soluble in acetone; NaCl and NaBr are not soluble in acetone. 63% 8-16 Relative Reactivity of Halide Leaving Groups Dr. Wolf's CHM 201 & 202 8-17 Generalization •Reactivity of halide leaving groups in nucleophilic substitution is the same as for elimination. RI most reactive RBr RCl RF Dr. Wolf's CHM 201 & 202 least reactive 8-18 Problem 8.2 A single organic product was obtained when 1-bromo-3-chloropropane was allowed to react with one molar equivalent of sodium cyanide in aqueous ethanol. What was this product? BrCH2CH2CH2Cl + NaCN Br is a better leaving group than Cl Dr. Wolf's CHM 201 & 202 8-19 Problem 8.2 A single organic product was obtained when 1-bromo-3-chloropropane was allowed to react with one molar equivalent of sodium cyanide in aqueous ethanol. What was this product? BrCH2CH2CH2Cl + NaCN :N Dr. Wolf's CHM 201 & 202 C CH2CH2CH2Cl + NaBr 8-20 The SN2 Mechanism of Nucleophilic Substitution Dr. Wolf's CHM 201 & 202 8-21 Kinetics •Many nucleophilic substitutions follow a second-order rate law. CH3Br + HO – CH3OH + Br – • rate = k[CH3Br][HO – ] • inference: rate-determining step is bimolecular Dr. Wolf's CHM 201 & 202 8-22 Bimolecular mechanism HO Br CH3 transition state HO – + CH3Br Dr. Wolf's CHM 201 & 202 •one step HOCH3 + Br – 8-23 Stereochemistry •Nucleophilic substitutions that exhibit second-order kinetic behavior are stereospecific and proceed with inversion of configuration. Dr. Wolf's CHM 201 & 202 8-24 Inversion of Configuration nucleophile attacks carbon from side opposite bond to the leaving group Dr. Wolf's CHM 201 & 202 three-dimensional arrangement of bonds in product is opposite to that of reactant 8-25 Stereospecific Reaction •A stereospecific reaction is one in which stereoisomeric starting materials give stereoisomeric products. •The reaction of 2-bromooctane with NaOH (in ethanol-water) is stereospecific. • (+)-2-Bromooctane (–)-2-Octanol • (–)-2-Bromooctane (+)-2-Octanol Dr. Wolf's CHM 201 & 202 8-26 Stereospecific Reaction H (CH ) CH 2 5 3 CH3(CH2)5 H NaOH C Br CH3 (S)-(+)-2-Bromooctane Dr. Wolf's CHM 201 & 202 HO C CH3 (R)-(–)-2-Octanol 8-27 Problem 8.4 The Fischer projection formula for (+)-2-bromooctane is shown. Write the Fischer projection of the(–)-2-octanol formed from it by nucleophilic substitution with inversion of configuration. CH3 H CH3 Br CH2(CH2)4CH3 Dr. Wolf's CHM 201 & 202 HO H CH2(CH2)4CH3 8-28 Steric Effects in SN2 Reactions Dr. Wolf's CHM 201 & 202 8-29 Crowding at the Reaction Site The rate of nucleophilic substitution by the SN2 mechanism is governed by steric effects. Crowding at the carbon that bears the leaving group slows the rate of bimolecular nucleophilic substitution. Dr. Wolf's CHM 201 & 202 8-30 Table 8.2 Reactivity toward substitution by the SN2 mechanism RBr + LiI RI + LiBr •Alkyl bromide Class Relative rate •CH3Br Methyl 221,000 •CH3CH2Br Primary 1,350 •(CH3)2CHBr Secondary 1 •(CH3)3CBr too small to measure Dr. Wolf's CHM 201 & 202 Tertiary 8-31 Decreasing SN2 Reactivity CH3Br CH3CH2Br (CH3)2CHBr (CH3)3CBr Dr. Wolf's CHM 201 & 202 8-32 Decreasing SN2 Reactivity CH3Br CH3CH2Br (CH3)2CHBr (CH3)3CBr Dr. Wolf's CHM 201 & 202 8-33 Crowding Adjacent to the Reaction Site The rate of nucleophilic substitution by the SN2 mechanism is governed by steric effects. Crowding at the carbon adjacent to the one that bears the leaving group also slows the rate of bimolecular nucleophilic substitution, but the effect is smaller. Dr. Wolf's CHM 201 & 202 8-34 Table 8.3 Effect of chain branching on rate of SN2 substitution RBr + LiI RI + LiBr •Alkyl bromide Structure Relative rate •Ethyl CH3CH2Br 1.0 •Propyl CH3CH2CH2Br 0.8 •Isobutyl (CH3)2CHCH2Br 0.036 •Neopentyl (CH3)3CCH2Br Dr. Wolf's CHM 201 & 202 0.00002 8-35 Nucleophiles and Nucleophilicity Dr. Wolf's CHM 201 & 202 8-36 Nucleophiles The nucleophiles described in Sections 8.1-8.6 have been anions. – .. – .. – .. – : etc. : N C: : : HS HO CH O 3 .. .. .. Not all nucleophiles are anions. Many are neutral. .. .. : NH3 for example CH3OH HOH .. .. All nucleophiles, however, are Lewis bases. Dr. Wolf's CHM 201 & 202 8-37 Nucleophiles Many of the solvents in which nucleophilic substitutions are carried out are themselves nucleophiles. .. HOH .. .. CH3OH .. for example The term solvolysis refers to a nucleophilic substitution in which the nucleophile is the solvent. Dr. Wolf's CHM 201 & 202 8-38 Solvolysis substitution by an anionic nucleophile R—X + :Nu— R—Nu + :X— solvolysis R—X + :Nu—H + R—Nu—H + :X— step in which nucleophilic substitution occurs Dr. Wolf's CHM 201 & 202 8-39 Solvolysis substitution by an anionic nucleophile R—X + :Nu— R—Nu + :X— solvolysis R—X + :Nu—H + R—Nu—H + :X— products of overall reaction R—Nu + HX Dr. Wolf's CHM 201 & 202 8-40 Example: Methanolysis Methanolysis is a nucleophilic substitution in which methanol acts as both the solvent and the nucleophile. CH3 R—X + : O: + R O: H H Dr. Wolf's CHM 201 & 202 CH3 CH3 –H+ R O .. : The product is a methyl ether. 8-41 Typical solvents in solvolysis solvent product from RX water (HOH) methanol (CH3OH) ethanol (CH3CH2OH) ROH ROCH3 ROCH2CH3 O O formic acid (HCOH) O acetic acid (CH3COH) Dr. Wolf's CHM 201 & 202 ROCH O ROCCH3 8-42 Nucleophilicity is a measure of the reactivity of a nucleophile. • Table 8.4 compares the relative rates of nucleophilic substitution of a variety of nucleophiles toward methyl iodide as the substrate. The standard of comparison is methanol, which is assigned a relative rate of 1.0. Dr. Wolf's CHM 201 & 202 8-43 Table 8.4 Nucleophilicity Rank Nucleophile strong good I-, HS-, RSBr-, HO-, RO-, CN-, N3NH3, Cl-, F-, RCO2H2O, ROH RCO2H fair weak very weak Dr. Wolf's CHM 201 & 202 Relative rate >105 104 103 1 10-2 8-44 Major factors that control nucleophilicity 1) basicity 2) solvation small negative ions are highly solvated in protic solvents large negative ions are less solvated 3) polarizability Dr. Wolf's CHM 201 & 202 8-45 Table 8.4 Nucleophilicity Rank Nucleophile Relative rate good HO–, RO– 104 RCO2– 103 H2O, ROH 1 fair weak When the attacking atom is the same (oxygen in this case), nucleophilicity increases with increasing basicity. Dr. Wolf's CHM 201 & 202 8-46 Major factors that control nucleophilicity 1) basicity 2) solvation small negative ions are highly solvated in protic solvents large negative ions are less solvated 3) polarizability Dr. Wolf's CHM 201 & 202 8-47 Table 8.4 Nucleophilicity Rank Nucleophile Relative rate strong I- >105 good Br- 104 fair Cl-, F- 103 A tight solvent shell around an ion makes it less reactive. Larger ions are less solvated than smaller ones and are more nucleophilic. Dr. Wolf's CHM 201 & 202 8-48 Major factors that control nucleophilicity 1) basicity 2) solvation small negative ions are highly solvated in protic solvents large negative ions are less solvated 3) polarizability Dr. Wolf's CHM 201 & 202 8-49 Table 8.4 Nucleophilicity Rank Nucleophile Relative reactivity strong I- >105 good Br- 104 fair Cl-, F- 103 More polarizable ions are more nucleophilic than less polarizable ones. Polarizability increases with increasing ionic size. Dr. Wolf's CHM 201 & 202 8-50 Unimolecular Nucleophilic Substitution SN1 Dr. Wolf's CHM 201 & 202 8-51 Tertiary alkyl halides are very unreactive in substitutions that proceed by the SN2 mechanism. But they do undergo nucleophilic substitution. But by a mechanism different from SN2. The most common examples are seen in solvolysis reactions. Dr. Wolf's CHM 201 & 202 8-52 Example of a solvolysis: Hydrolysis of tert-butyl bromide CH3 CH3 C H .. Br : .. + : O: H CH3 CH3 CH3 C .. OH .. + H .. Br : .. CH3 Dr. Wolf's CHM 201 & 202 8-53 Example of a solvolysis: Hydrolysis of tert-butyl bromide CH3 CH3 C H .. Br : .. + CH3 : O: H + O: C H CH3 CH3 H CH3 + CH3 CH3 C .. OH .. + H .. Br : .. .. – : Br : .. CH3 Dr. Wolf's CHM 201 & 202 8-54 Example of a solvolysis: Hydrolysis of tert-butyl bromide CH3 CH3 C CH3 H .. Br : .. + : O: CH3 CH3 H + O: C H H CH3 + This is the nucleophilic substitution stage of the reaction; the one with which we are concerned. Dr. Wolf's CHM 201 & 202 .. – : Br : .. 8-55 Example of a solvolysis: Hydrolysis of tert-butyl bromide CH3 CH3 C CH3 H .. Br : .. + CH3 : O: CH3 H + O: C H H CH3 + The reaction rate is independent of the concentration of the nucleophile and follows a first-order rate law. rate = k[(CH3)3CBr] Dr. Wolf's CHM 201 & 202 .. – : Br : .. 8-56 Example of a solvolysis: Hydrolysis of tert-butyl bromide CH3 CH3 C CH3 H .. Br : .. + CH3 : O: CH3 H + O: C H H CH3 + The mechanism of this step is not SN2. It is called SN1 and begins with ionization of (CH3)3CBr. Dr. Wolf's CHM 201 & 202 .. – : Br : .. 8-57 rate = k[alkyl halide] First-order kinetics implies a unimolecular rate-determining step. Proposed mechanism is called SN1, which stands for substitution nucleophilic unimolecular Dr. Wolf's CHM 201 & 202 8-58 CH3 CH3 .. Br : .. C CH3 unimolecular slow H3C + C CH3 + .. – : Br : .. CH3 Dr. Wolf's CHM 201 & 202 8-59 H3C CH3 + C H : O: CH3 H bimolecular fast CH3 CH3 H + C CH3 Dr. Wolf's CHM 201 & 202 O: H 8-60 carbocation formation carbocation capture R+ proton transfer RX + ROH2 Dr. Wolf's CHM 201 & 202 ROH 8-61 Characteristics of the SN1 mechanism first order kinetics: rate = k[RX] unimolecular rate-determining step carbocation intermediate rate follows carbocation stability rearrangements sometimes observed reaction is not stereospecific much racemization in reactions of optically active alkyl halides Dr. Wolf's CHM 201 & 202 8-62 The rate of nucleophilic substitution by the SN1 mechanism is governed by electronic effects. Carbocation formation is rate-determining. The more stable the carbocation, the faster its rate of formation, and the greater the rate of unimolecular nucleophilic substitution. Dr. Wolf's CHM 201 & 202 8-63 Table 8.5 Reactivity toward substitution by the SN1 mechanism RBr solvolysis in aqueous formic acid Alkyl bromide Class Relative rate CH3Br Methyl 1 CH3CH2Br Primary 2 (CH3)2CHBr Secondary (CH3)3CBr Tertiary Dr. Wolf's CHM 201 & 202 43 100,000,000 8-64 Decreasing SN1 reactivity (CH3)3CBr (CH3)2CHBr CH3CH2Br Dr. Wolf's CHM 201 & 202 CH3Br 8-65 Stereochemistry of SN1 Reactions Dr. Wolf's CHM 201 & 202 8-66 Nucleophilic substitutions that exhibit first-order kinetic behavior are not stereospecific. Dr. Wolf's CHM 201 & 202 8-67 Stereochemistry of an SN1 Reaction CH3 H C R-(–)-2-Bromooctane Br CH3(CH2)5 H HO CH3 CH3 H2O H C OH C (CH2)5CH3 (S)-(+)-2-Octanol (83%) Dr. Wolf's CHM 201 & 202 CH3(CH2)5 (R)-(–)-2-Octanol (17%) 8-68 Figure 8.8 Ionization step gives carbocation; three bonds to stereogenic center become coplanar + – Dr. Wolf's CHM 201 & 202 8-69 Figure 8.8 + – Leaving group shields one face of carbocation; nucleophile attacks faster at opposite face. Dr. Wolf's CHM 201 & 202 8-70 Figure 8.8 – + – – More than 50% Dr. Wolf's CHM 201 & 202 Less than 50% 8-71 Carbocation Rearrangements in SN1 Reactions Dr. Wolf's CHM 201 & 202 8-72 Because carbocations are intermediates in SN1 reactions, rearrangements are possible. Dr. Wolf's CHM 201 & 202 8-73 Example CH3 CH3 CH3 H2O C CHCH3 H Br Dr. Wolf's CHM 201 & 202 CH3 C OH CH2CH3 (93%) 8-74 Example CH3 CH3 CH3 C CHCH3 H Br CH3 C CH2CH3 OH (93%) H2O CH3 CH3 C H Dr. Wolf's CHM 201 & 202 CH3 CHCH3 + CH3 C + CHCH3 H 8-75 Solvent Effects Dr. Wolf's CHM 201 & 202 8-76 SN1 Reaction Rates Increase in Polar Solvents Dr. Wolf's CHM 201 & 202 8-77 Table 8.6 SN1 Reactivity versus Solvent Polarity Solvent acetic acid methanol formic acid water Dielectric constant Relative rate 6 33 58 78 1 4 5,000 150,000 Most polar Dr. Wolf's CHM 201 & 202 Fastest rate 8-78 transition state stabilized by polar solvent + R X R+ energy of RX not much affected by polarity of RX solvent Dr. Wolf's CHM 201 & 202 8-79 transition state stabilized by polar solvent activation energy decreases; rate increases + R X R+ energy of RX not much affected by polarity of RX solvent Dr. Wolf's CHM 201 & 202 8-80 SN2 Reaction Rates Increase in Polar Aprotic Solvents An aprotic solvent is one that does not have an —OH group. So it does not solvate anion well allowing it to be effective nucleophile Dr. Wolf's CHM 201 & 202 8-81 Table 8.7 SN2 Reactivity versus Type Solvent Table 8.7 SN2 Reactivity vs of Solvent CH3CH2CH2CH2Br + N3– Solvent CH3OH H2O DMSO DMF Acetonitrile Dr. Wolf's CHM 201 & 202 Type polar protic polar protic polar aprotic polar aprotic polar aprotic Relative rate 1 7 1300 2800 5000 8-82 Mechanism Summary SN1 and SN2 Dr. Wolf's CHM 201 & 202 8-83 When... primary alkyl halides undergo nucleophilic substitution, they always react by the SN2 mechanism tertiary alkyl halides undergo nucleophilic substitution, they always react by the SN1 mechanism secondary alkyl halides undergo nucleophilic substitution, they react by the SN1 mechanism in the presence of a weak nucleophile (solvolysis) SN2 mechanism in the presence of a good nucleophile Dr. Wolf's CHM 201 & 202 8-84 Substitution And Elimination As Competing Reactions Dr. Wolf's CHM 201 & 202 8-85 We have seen that alkyl halides can react with Lewis bases in two different ways. They can undergo nucleophilic substitution or elimination. b-elimination C H C C + :Y X C + H Y + :X – – H C C + :X – Y Dr. Wolf's CHM 201 & 202 nucleophilic substitution 8-86 How can we tell which reaction pathway is followed for a particular alkyl halide? b-elimination C H C C + :Y X C + H Y + :X – – H C C + :X – Y Dr. Wolf's CHM 201 & 202 nucleophilic substitution 8-87 A systematic approach is to choose as a reference point the reaction followed by a typical alkyl halide (secondary) with a typical Lewis base (an alkoxide ion). The major reaction of a secondary alkyl halide with an alkoxide ion is elimination by the E2 mechanism. Dr. Wolf's CHM 201 & 202 8-88 Example CH3CHCH3 Br NaOCH2CH3 ethanol, 55°C CH3CHCH3 + CH3CH=CH2 OCH2CH3 (13%) Dr. Wolf's CHM 201 & 202 (87%) 8-89 Figure 8.11 E2 CH3CH2 Dr. Wolf's CHM 201 & 202 .. O: .. – Br 8-90 Figure 8.11 SN2 CH3CH2 Dr. Wolf's CHM 201 & 202 ..– O: .. Br 8-91 Given that the major reaction of a secondary alkyl halide with an alkoxide ion is elimination by the E2 mechanism, we can expect the proportion of substitution to increase with: 1) decreased crowding at the carbon that bears the leaving group Dr. Wolf's CHM 201 & 202 8-92 Decreased crowding at carbon that bears the leaving group increases substitution relative to elimination. primary alkyl halide CH3CH2CH2Br NaOCH2CH3 ethanol, 55°C CH3CH2CH2OCH2CH3 + (91%) Dr. Wolf's CHM 201 & 202 CH3CH=CH2 (9%) 8-93 But a crowded alkoxide base can favor elimination even with a primary alkyl halide. primary alkyl halide + bulky base CH3(CH2)15CH2CH2Br KOC(CH3)3 tert-butyl alcohol, 40°C CH3(CH2)15CH2CH2OC(CH3)3 + CH3(CH2)15CH=CH2 (13%) Dr. Wolf's CHM 201 & 202 (87%) 8-94 Given that the major reaction of a secondary alkyl halide with an alkoxide ion is elimination by the E2 mechanism, we can expect the proportion of substitution to increase with: 1) decreased crowding at the carbon that bears the leaving group 2) decreased basicity of nucleophile Dr. Wolf's CHM 201 & 202 8-95 Weakly basic nucleophile increases substitution relative to elimination secondary alkyl halide + weakly basic nucleophile CH3CH(CH2)5CH3 Cl KCN SN2 pKa (HCN) = 9.1 DMSO CH3CH(CH2)5CH3 CN (70%) Dr. Wolf's CHM 201 & 202 8-96 Weakly basic nucleophile increases substitution relative to elimination secondary alkyl halide + weakly basic nucleophile I SN2 pKa (HN3) = 4.6 NaN3 (even weaker base) N3 (75%) Dr. Wolf's CHM 201 & 202 8-97 Tertiary alkyl halides are so sterically hindered that elimination is the major reaction with all anionic nucleophiles. Only in solvolysis reactions does substitution predominate over elimination with tertiary alkyl halides. Dr. Wolf's CHM 201 & 202 8-98 (CH3)2CCH2CH3 Example Br CH3 CH3CCH2CH3 CH3 + CH2=CCH2CH3 + CH3 CH3C=CHCH3 OCH2CH3 ethanol, 25°C 36% 64% 2M sodium ethoxide in ethanol, 25°C 1% 99% Dr. Wolf's CHM 201 & 202 8-99 Mechanism Summary SN1 and SN2 and E1 and E2 Under 2nd order conditions….. STRONG base/nucleophile eg. -OH, -OR ELIMINATION favored with 30 , 20, (and 10 with bulky base eg. -OtBu) SUBSTITUTION favored with 10 (aprotic solvent helps) With WEAK base but good nucleophile e.g. -CN, -N3 Or Under 1st order conditions….. WEAK base/nucleophile (solvolysis) e.g. H2O, ROH, SUBSTITUTION favored (increased solvent polarity helps) Dr. Wolf's CHM 201 & 202 8-100 Nucleophilic Substitution of Alkyl Sulfonates Dr. Wolf's CHM 201 & 202 8-101 Leaving Groups • we have seen numerous examples of nucleophilic substitution in which X in RX is a halogen • halogen is not the only possible leaving group though Dr. Wolf's CHM 201 & 202 8-102 Other RX compounds O O HOSOH ROSCH3 O O Sulfuric acid O ROS Alkyl methanesulfonate (mesylate) CH3 O Alkyl p-toluenesulfonate (tosylate) • undergo same kinds of reactions as alkyl halides Dr. Wolf's CHM 201 & 202 8-103 Preparation Tosylates are prepared by the reaction of alcohols with p-toluenesulfonyl chloride (usually in the presence of pyridine) ROH + CH3 SO2Cl pyridine O ROS CH3 • (abbreviated as ROTs) O Dr. Wolf's CHM 201 & 202 8-104 Tosylates undergo typical nucleophilic substitution reactions H KCN H CH2OTs ethanolwater CH2CN (86%) SN2 Dr. Wolf's CHM 201 & 202 8-105 •The best leaving groups are weakly basic Dr. Wolf's CHM 201 & 202 8-106 Table 8.8 Approximate Relative Reactivity of Leaving Groups •Leaving GroupRelative Conjugate acid Ka of Rate of leaving group conj. acid • F– 10-5 HF 3.5 x 10-4 wk acid • Cl– 1 HCl 107 • Br– 10 HBr 109 • I– 102 HI 1010 • H2O 101 H3O+ 56 • TsO– 105 TsOH 600 • CF3SO2O– 108 CF3SO2OH 106 Dr. Wolf's CHM 201 & 202 8-107 Table 8.8 Approximate Relative Reactivity of Leaving Groups •Leaving GroupRelative Conjugate acid Ka of Rate of leaving group conj. acid • F– 10-5 HF 3.5 x 10-4 • Cl– 1 HCl 107 Sulfonate esters are extremely good leaving groups; 9 •sulfonate Br– ions are 10 very weakHBr 10 bases. • I– 102 HI 1010 • H2O 101 H3O+ 56 • TsO– 105 TsOH 600 • CF3SO2O– 108 CF3SO2OH 106 Dr. Wolf's CHM 201 & 202 8-108 Tosylates can be converted to alkyl halides CH3CHCH2CH3 OTs NaBr DMSO SN2 CH3CHCH2CH3 Br (82%) • Tosylate is a better leaving group than bromide. Dr. Wolf's CHM 201 & 202 8-109 Tosylates allow control of stereochemistry • Preparation of tosylate does not affect any of the bonds to the stereogenic center, so configuration and optical purity of tosylate is the same as the alcohol from which it was formed. H H CH3(CH2)5 TsCl C CH3(CH2)5 C OH OTs pyridine H3C Dr. Wolf's CHM 201 & 202 H3C 8-110 Tosylates allow control of stereochemistry • Having a tosylate of known optical purity and absolute configuration then allows the preparation of other compounds of known configuration by SN2 processes. H H CH3(CH2)5 C Nu– OTs (CH2)5CH3 Nu C SN2 H3C Dr. Wolf's CHM 201 & 202 CH3 8-111 Looking Back: Reactions of Alcohols with Hydrogen Halides Dr. Wolf's CHM 201 & 202 8-112 Secondary alcohols react with hydrogen halides with net inversion of configuration H CH3 Br C 87% H H3C (CH2)5CH3 HBr C CH3(CH2)5 OH H 13% Since some racemization, can’t be SN2 Dr. Wolf's CHM 201 & 202 H3C C Br CH3(CH2)5 8-113 Secondary alcohols react with hydrogen halides with net inversion of configuration H • H CH3 Br C 87% (CH2)5CH3 H3C Most reasonableHBr mechanism is SN1 with front side of C shielded carbocation by leaving group OH CH3(CH2)5 H 13% H3C C Br CH3(CH2)5 Dr. Wolf's CHM 201 & 202 8-114 Rearrangements can occur in the reaction of alcohols with hydrogen halides OH HBr Br + Br 93% Dr. Wolf's CHM 201 & 202 7% 8-115 Rearrangements HBr OH 7% + + 93% Br – Br Br – + Br Dr. Wolf's CHM 201 & 202 8-116 End of Chapter 8 Dr. Wolf's CHM 201 & 202 8-117